ABSTRACT
INTRODUCTION Many clinical settings lack the necessary resources to complete angiographic studies, which are commonly used to predict complications and death following acute coronary syndrome. Corrected QT-interval dispersion can be useful for assessing risk of myocardial infarction recurrence.
OBJECTIVE Evaluate the relationship between corrected QT-interval dispersion and recurrence of myocardial infarction in patients with ST-segment elevation.
METHODS We conducted a prospective observational study of 522 patients with ST-segment elevation myocardial infarction admitted consecutively to the Camilo Cienfuegos General Provincial Hospital in Sancti Spiritus, Cuba, from January 2014 through June 2017. Of these, 476 were studied and 46 were excluded because they had other disorders. Demographic variables and classic cardiovascular risk factors were included. Blood pressure, heart rate, blood glucose, and corrected and uncorrected QT-interval duration and dispersion were measured. Patients were categorized according to the Killip-Kimball classification. Association between dispersion of the corrected QT-interval and recurrence of infarction was analyzed using a binary logistic regression model, a regression tree and receiver operator characteristic curves.
RESULTS Patients with recurrent infarction (56; 11.8%) had higher average initial blood glucose values than those who did not have recurrence; the opposite occurred for systolic and diastolic blood pressure and for left ventricular ejection fraction. Dispersion of the corrected QT-interval was a good predictor of infarction recurrence according to a multivariate analysis (OR = 3.09; 95% CI = 1.105–8.641; p = 0.032). Cardiac arrest is the variable that best predicts recurrence. No recurrence of infarction occurred in 97% of patients without cardiac arrest, left ventricular ejection fraction >45% and corrected QT-interval dispersion <80 ms.
CONCLUSIONS Risk of infarction recurrence is low in patients without cardiac arrest, with left ventricular ejection fraction >45% and with dispersion of corrected QT-interval <80 ms. Patients with corrected QT-interval dispersion ≥80 ms have greater risk of recurrence of infarction, which suggests that this variable could be used for stratification of risk following ST-segment elevation myocardial infarction.
KEYWORDS ST-elevation myocardial infarction, myocardial infarction, electrocardiography, chronic disease, risk assessment, Cuba
INTRODUCTION
Cardiovascular diseases are the most frequent cause of death worldwide. Eighty percent of deaths due to heart attacks occur in middle- and low-income countries.[1] In Cuba, the heart disease mortality rate was 241.6 per 100,000 population in 2017; the rate for ischemic heart disease was 156.7, including mortality of chronic cases and from acute episodes; mortality rate from myocardial infarction (MI) was 71 per 100,000 population. In 2017, Sancti Spíritus Province had a crude mortality rate from heart disease of 231 per 100,000 population, age adjusted to 100.8 per 100,000 population.[2]
Among patients with acute coronary syndrome (ACS) the proportion with ST-segment elevation myocardial infarction (STEMI) ranges from 29% to 47%. Moreover, STEMI is the most severe of MI.[3] Although STEMI frequency is generally decreasing,[3] risk of death and complications following a STEMI is high despite diagnostic and treatment advances. In-hospital fatality varies from 4% to 12% for European Union countries, where 1-year mortality among STEMI patients is 10%.[4,5] Post-STEMI readmission rates are high, about 15.4%, with 26.6% of readmissions due to recurrent ischemia.[6]
IMPORTANCE Easily measured with equipment readily available even in low-resource clinical settings, corrected QT-interval dispersion following ST-elevation myocardial infarction offers a reliable and simple predictive tool for assessing myocardial infarction recurrence risk.
Prognosis for STEMI patients relates to the probability of developing short- or long-term complications and depends more on conditions upon admission than on prior coronary risk factors.[7–10] According to international MI treatment guidelines, conditions associated with poor outcomes are advanced age, development of some degree of heart failure, decreased ventricular function, diabetes, treatment strategy and type of hospital where the patient is treated.[5,11] Brogan[12] describes multiple models for stratifying risk of death and complications following MI that include variables such as troponin levels and coronary angiography data, but these are not always available in internal medicine and cardiology services in middle- and low-income countries.[13,14]
Acute myocardial ischemia changes QT-interval (QTi) duration. Although the causal mechanisms are controversial, a rise in repolarization heterogeneity of the ventricular myocardium increases the difference between maximum and minimum QTi, referred to as QT-interval dispersion (QTd).[15,16] QTi is measured by electrocardiogram (ECG) from initiation of the QRS complex to the point where the T wave returns to the isoelectric line. This interval corresponds to potential action duration, and includes ventricular depolarization and repolarization.[16–18] Corrected QTi (QTc) is the duration of this parameter, adjusted for heart rate.[17]
The dispersion of corrected QT-interval (QTdc) measures severity of coronary artery damage.[19–22] Values >59 ms have been associated with myocardial viability,[23] which makes QTdc a plausible predictor of MI recurrence. Higher QTdc is related to complications such as malignant ventricular arrhythmias,[24–26] but its relationship to recurrent ischemia has been less studied.
In a 2007 study, Kenigsberg[27] modified the classic ischemic cascade concept by demonstrating that the first indication of coronary occlusion is QTc prolongation. Acute ischemia causes an increase in potassium concentrations and shortening of repolarization time that leads to slow conduction and decreased excitability. Response to this damage is greater in the subepicardium than in the subendocardium and causes repolarization dispersion. Lack of homogeneity and increased spatial dispersion of repolarization results in increased QTdc in patients with ischemic heart disease.[16,28] Such physiological and pathological effects of acute ischemia support using QTdc as an important predictor of MI recurrence. Moreover, QTdc is obtained from surface ECG and is a simple, low-cost tool that can be useful in assessing risk of MI recurrence in STEMI cases, especially in low- and middle-income countries.
Recurrent MI, defined as a repetition of the signs and symptoms of acute heart failure in the first 28 days following an initial MI, carries a worse prognosis, including increased risk of death.[29] In recurrent MI, there is reocclusion of the affected artery, whether from initial non-reperfusion or associated with thrombosis from an implanted stent. Nowinski[30] demonstrated that when inflating the balloon during percutaneous coronary intervention (PCI) in patients with myocardial ischemia, immediate changes occur in ventricular repolarization and QTi is prolonged. Such changes persist for minutes and even hours. These findings suggested that QTi could be used as an early marker of acute and transitory myocardial ischemia, easily detected on a surface ECG, and useful for prognosis in middle- and low-income countries where therapeutic and diagnostic alternatives described in international guidelines are not always available.[4,11]
The objective of this paper is to evaluate the relationship between QTdc and MI recurrence in patients with ST-segment elevation myocardial infarction.
METHODS
Design and study population We conducted a prospective observational study of all STEMI patients admitted to the coronary care unit of Camilo Cienfuegos General Provincial Hospital (HGPCC) in Sancti Spíritus, Cuba, January 1, 2014 through June 30, 2017. The study enrolled 522 patients, of which 46 were excluded later for the following reasons: 13 for left bundle branch block; 11 for previous atrial fibrillation; 14 receiving pharmacological treatments that prolong QTi; and 8 with life expectancy less than 1 year due to non-cardiac conditions that could trigger MI recurrence. The final study group consisted of 476 patients with an average age of 67.4 years (SD = 13.8); 304 (63.9%) were men.
STEMI was diagnosed by pain typical of heart failure with new ST-segment elevation >0.2 mV, measured from point J on ≥2 precordial leads, or 0.1 mV on ≥2 standard leads.[4,11,29] Recurrence was diagnosed by the same criteria within the first 28 days following initial MI.[29]
Variables Demographic variables were age, sex and skin color (white, mestizo/mixed or black). Cardiovascular risk factors were hypertension (HT), prior ischemic heart disease, lipid metabolism disorders (cholesterol >6.71 mmol/L and triglycerides >1.60 mmol/L in women and >1.88 mmol/L in men, according to established reference values), smoking, history of diabetes, and obesity (defined as body mass index >30 kg/m2). Clinical variables were systolic and diastolic blood pressure (BP) and heart rate on admission.
The Killip-Kimball[31] classification was used to assess the degree of acute heart failure according to the following criteria:
- Class I. No heart failure. No clinical signs of cardiac decompensation.
- Class II. Heart failure. Diagnostic criteria include rales, third heart sound gallop and pulmonary venous HT, and pulmonary congestion with wet rales in the lower half of the lung fields.
- Class III. Severe heart failure. Obvious pulmonary edema with rales in all lung fields.
- Class IV. Cardiogenic shock. Clinical signs include hypotension (systolic BP <90 mmHg) and evidence of peripheral vasoconstriction, such as oliguria, cyanosis and sweating.
Laboratory variables were hemoglobin, blood glucose, leukogram, creatinine and creatine kinase (CPK); CPK was repeated at 6, 12, 24 and 48 hours, and the maximum value was used. Venous blood samples taken within 24 hours of patient admission during initial MI were processed with the High Technologies COBAS c311 automated analyzer (Hitachi,Tokyo, Japan).
Reperfusion strategy was thrombolysis with 1,500,000 IU intravenous Heberkinasa (recombinant streptokinase, Heber Biotec SA, Cuba).[32] In no case was primary coronary intervention performed as established by international guidelines for MI treatment, as no hemodynamic service was available.[4,11] Infarction location was determined by admission ECG and classified using Bayés de Luna’s criteria (large anterior, mid-anterior, apical anterior, septal, inferior, inferolateral and lateral wall).[33] Complications studied were new-onset atrial fibrillation determined by surface ECG, cardiac arrest on admission, and death. Once hemodynamic stability was attained without signs of hypotension, extreme bradycardia or arrhythmias that could endanger the patient’s life, a transthoracic echocardiogram was performed using the ProSound Alpha 5 (ALOKA, Japan), and left ventricular ejection fraction (LVEF) was determined by the biplane Simpson method.[34]
Electrocardiographic variables A 12-lead ECG was performed upon admission before thrombolysis, repeated after 90 minutes and then every hour for the first 6 hours. Electrocardiographic variables were based on the first ECG in non-thrombolysed cases and on the 90-minute ECG in the other patients. ECG was recorded at a sweep speed of 25 mm/s with 10 mm/mV standardization using a CardiocidBB electrocardiograph (Central Digital Research Institute, Cuba)[35] with a band-pass filter that restricts frequencies to a spectrum of 0.05–150 Hz, and a comb filter for hum at 60 Hz. Two observers manually and independently measured the following parameters on all ECG leads with a magnifying lens:[19,20,36]
- QTi: QT interval, corresponding to the time in milliseconds from initiation of QRS complex to T wave termination, defined as the point when the T wave returns to the isoelectric line, or the nadir between T and U waves whenever the latter was present.[16,17] It was measured for all leads and the average calculated;
- QTc: QT interval corrected following Bazett’s formula;[18]
- QTd: Difference between the maximum and minimum QTi measured on 12 ECG leads; and
- QTdc: Difference between corrected maximum and minimum QTi measured on the 12 ECG leads.
Data collection settings and procedures Patient assessment and followup were carried out by cardiologists. Hospital stays lasted five to seven days. Followup was done for one month after discharge, with hospital outpatient visits on days 15 and 28. MI recurrence was diagnosed during this period. Data were collected on forms that included study variables.
Data collection settings and procedures Patient assessment and followup were carried out by cardiologists. Hospital stays lasted five to seven days. Followup was done for one month after discharge, with hospital outpatient visits on days 15 and 28. MI recurrence was diagnosed during this period. Data were collected on forms that included study variables.
To verify strength of association among qualitative variables (sex, skin color, risk factors, Killip-Kimball class, reperfusion strategy and complications), the non-parametric Pearson chi square test was used. To measure association between a continuous quantitative variable (QTdc) and an ordinal qualitative variable (Killip-Kimball class), the Spearman correlation coefficient was used. For all statistical tests, a significance threshold of p = 0.05 was applied.
To obtain the QTdc cutoff point with best metric properties (sensitivity and specificity), a receiver operator characteristic curve was constructed. With these results, a value of 80 ms was determined and used to dichotomize the variable and include it in a logistic regression model together with other binary predictors (cardiac arrest, systolic BP ≤100 mmHg, LVEF ≤45%, Killip-Kimball Class II- IV, blood glucose ≥11 mmol/L, and large anterior MI). Epidat 3.1 statistical software was used to calculate sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for MI recurrence of a QTdc greater or lesser than 80 ms.
To assess the independent role of QTdc in prediction of recurrent MI, a binary logistic regression model was fitted, in which MI recurrence was considered the dependent (dichotomous) variable. Estimated coefficients were expressed as odds ratios (OR) with their respective 95% confidence intervals (95% CI). For inclusion of covariates in the logistic regression model together with QTdc, three criteria were applied: clinical or anatomic functional significance, statistical significance in the prior bivariate analysis and a principle of parsimony (Occam’s razor) to prevent inclusion of redundant variables. The variables included were cardiac arrest on admission, QTdc, systolic BP, LVEF, Killip-Kimball class, location of infarction and blood glucose. To identify whether QTdc contributes predictive capacity when cardiac arrest has occurred and LVEF values are <45%, a regression tree model was constructed using variables included in the logistic model.
Ethics The study design respected the Helsinki Declaration principles[37] and was approved by the HGPCC’s ethics committee. Each patient was informed of the study details and their written consent was obtained; in extremely serious cases or loss of consciousness, an immediate relative provided written informed consent. The study design did not include manipulation of variables, and the hospital’s established MI treatment protocol was observed. To respect privacy and confidentiality, databases used coded information, without names or patient identifiers.
RESULTS
MI recurrence was seen in 56 (11.8%) patients; 17 (30.4%) had recurrence before hospital discharge, and the remainder within the following 28 days. Average age and sex distribution were similar in both groups. Relative frequency of recurrence did not differ by skin color. Nor did cardiovascular risk factors (HT, diabetes, dyslipidemia, tobacco use, prior ischemic heart disease and obesity) differ between patients with and without MI recurrence.
Reperfusion by thrombolysis was performed in 82.1% of patients without MI recurrence and in 76.8% of patients with recurrence. Thrombolysis was not used in 88 patients for various reasons: 41 (46.6%), prolonged ischemia; 12 (13.6%), recent use of Heberkinasa; 11 (12.5%), transient ischemic attack in the preceding 6 months; 9 (10.2%), cerebrovascular accident; 6 (6.8%), refractory cardiogenic shock; 5 (5.7%), known bleeding disorders; and 4 (4.5%), gastrointestinal bleeding in the previous month. Although not included in Table 1, this distribution was similar in both patient groups.
Mean blood glucose on admission was higher in patients with recurrence of acute coronary syndrome (ACS). Mean BP and mean LVEF recorded during admission were lower in this group of patients. In patients with recurrent MI, atrial fibrillation and cardiac arrest were frequent complications and mortality was significantly higher. The most frequent initial infarction location for patients with recurrent MI was the large anterior myocardium, while the inferior myocardium was the most frequent location for those without recurrent MI.(Table 1).
A positive correlation was observed between degree of heart failure (Killip-Kimball class) and QTdc (Spearman rho 0.697, p ≤0.001). Patients with cardiac arrest had higher QTdc means (m = 94.7, SD = 30.9) compared to the others (m = 63.9, SD = 29.5) with p = 0.001. Cases with recurrent ischemia and cardiac arrest also had higher QTdc means (m = 105.0, SD = 22.3) when compared to those without cardiac arrest (m = 75.7, SD = 21.2).(Table 2).
Calculation of covariate-adjusted ORs based on the logistic regression model showed a significant association between QTdc and MI recurrence (OR = 3.09; 95% CI = 1.105 – 8.641; p = 0.032) which, although much lower than that attributable to cardiac arrest history (OR = 51.22; 95% CI = 16.72–156.97), suggests a marginal predictive effect for QTdc.(Table 3).
The QTdc cutoff point had a sensitivity of 66.1% and specificity of 68.1%. (Table 4) The probability of infarction not recurring in patients with QTdc <80 ms is higher (NPV = 93.8%) than the probability of recurrence in patients with QTdc ≥80 ms (PPV = 21.9%).
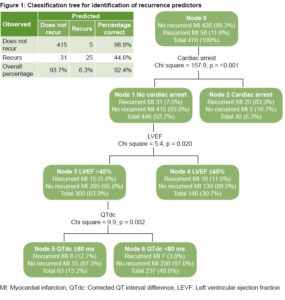
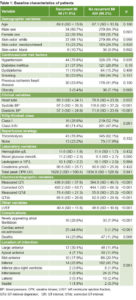 The regression tree model showed that cardiac arrest is the variable with greatest predictive capacity for MI recurrence. In cases that did not experience cardiac arrest (446 patients, 93.7%), LVEF >45% was an important predictor of non-recurrence. Ninety-seven percent of patients without cardiac arrest, with LVEF >45% and QTdc <80 ms did not have MI recurrence. This model correctly classified 92.4% of cases overall (sensitivity 44.6%; specificity 98.8%).(Figure 1).
The regression tree model showed that cardiac arrest is the variable with greatest predictive capacity for MI recurrence. In cases that did not experience cardiac arrest (446 patients, 93.7%), LVEF >45% was an important predictor of non-recurrence. Ninety-seven percent of patients without cardiac arrest, with LVEF >45% and QTdc <80 ms did not have MI recurrence. This model correctly classified 92.4% of cases overall (sensitivity 44.6%; specificity 98.8%).(Figure 1).
DISCUSSION
QT is an electrocardiographic indicator of regional differences and their heterogeneity during cardiac repolarization.[16,18] QTdc is a predictor of ventricular arrhythmias,[24–26] an indicator of myocardial viability[23,38-40] and more recently, it has been considered an indicator of successful reperfusion and associated with greater severity of coronary artery disease (CAD).[19–22,41]
Since myocardial ischemia occurs in viable tissue with significant CAD, QTdc should be a good predictor of MI recurrence. However, no studies were found assessing its prognostic capacity.
Jensen[39] demonstrated a QTdc decrease following recanalization of the affected artery and George[40] found a greater reduction of this electrocardiographic parameter following PCI as compared to fibrinolysis. Eslami[41] also demonstrated a significant QTdc reduction following PCI (5.8 ms mean compared with 3.6 ms, p <0.001). These studies showed that when the artery is successfully opened through primary coronary intervention—the suggested treatment in international guidelines—[1,9] ventricular repolarization homogeneity is reestablished between the affected myocardium’s different zones. QTdc values found in this study suggest absence of flow reestablishment in the artery responsible for infarction.
 This study’s results show high QTdc values, which were higher in patients with MI recurrence. This coincides with Pekdemir,[42] who demonstrated a relationship between QTd >40 ms and appearance of new ACS and death, despite a normal initial ECG. Furthermore, Machín[43] found infarction recurrence within 30 days following initial MI in 26% of cases, of which 86% presented increased QTd with a significant association (p = 0.009).
This study’s results show high QTdc values, which were higher in patients with MI recurrence. This coincides with Pekdemir,[42] who demonstrated a relationship between QTd >40 ms and appearance of new ACS and death, despite a normal initial ECG. Furthermore, Machín[43] found infarction recurrence within 30 days following initial MI in 26% of cases, of which 86% presented increased QTd with a significant association (p = 0.009).
QTdc has also been associated with myocardial viability, a necessary condition for recurrence of angina. Once the necrotic scar forms, recurring ischemic episodes are very unlikely. Using low-dose dobutamine (10 mg), Moreno[23] found significant differences in QTdc between patients with viable and nonviable myocardium (m = 86.1, SD = 30.8 and m = 60.0, SD = 20.1 ms respectively; p = 0.013) and concluded that a QTdc >59 ms predicts greater myocardial viability. Ikonomidis[44] and Lancellotti[45] also found higher QTd in patients with viable myocardium. If these results are considered, it can be assumed that STEMI patients with QTdc ≥80 ms also presented viable myocardium.
High QTdc values have been associated with greater severity of coronary disease. Akgumus found significantly higher QTdc in patients with 3-vessel disease than in patients with 1-vessel disease (m = 68, SD = 32 and m = 50, SD 32 ms; respectively; p = 0.001).[20] In another study, however, relating severity of CAD to this electrocardiographic parameter in patients with chronic ischemic heart disease, Stankovic found higher values in patients with affected vessels as compared to those with three affected vessels.[19]
Several factors have been associated with greater QTdc during acute ischemia. Thus, it is uncertain whether higher QTdc predicts higher risk for ischemic patients or is the expression of other cardiovascular risk factors such as hyperglycemia, obesity and left ventricular hypertrophy.[19] The results of this study show an association between Killip-Kimball class and greater QTdc, both of which are evidence of a greater degree of heart failure. In a retrospective study, Chávez-González[46] found the variables most associated with a QTdc >50 ms were ischemic heart disease (OR 4.2; 95% CI 1.84–10.13; p = 0.001), hypertension (OR 3.56; 95% CI 1.73–7.34; p = 0.001) and diabetes mellitus (OR 3.21; 95% CI 1.46–7.05; p = 0.002), which supports a hypothesis of association of greater morbidity with greater repolarization dispersion.
Mortality in our study population was higher in patients with MI recurrence, consistent with findings by Jiménez-Candil[47] who included patients with non-ST-segment elevation ACS. This author found that QTc ≥450 ms was a predictor of independent risk of death or recurrent ischemia (adjusted OR 3.8; 95% CI 2.5–6.5; p <0.001). Another study conducted in Santa Clara, Villa Clara Province, Cuba by Rodríguez González[48] found QTdc >50 ms associated with greater mortality and incidence of a new ACS within 30 days of hospital discharge. Our results suggest the importance of evaluating QTdc with risk stratification following STEMI, especially in patients without cardiac arrest on admission and with LVEF >45%, which characterized most patients in this study. A study limitation was that primary percutaneous coronary intervention was not performed and therefore it was not possible to correlate QTdc values with the severity of coronary disease. Nevertheless, these results could be useful for low- and middle-income countries in need of quality, low-cost medical care alternatives.
CONCLUSIONS
Risk of infarction recurrence is low in patients without cardiac arrest, with left ventricular ejection fraction >45% and with dispersion of corrected QT-interval <80 ms. Patients with QTdc ≥80 ms have a greater risk of MI recurrence, which suggests the utility of this parameter for risk stratification after STEMI in settings with limited resources.
References

- Anderson JL, Morrow DA. Acute myocardial infarction. N Engl J Med. 2017 May 25;376(21):2053–64.
- National Health Statistics and Medical Records Division (CU). Anuario Estadístico de Salud 2017 [Internet]. Havana: Ministry of Public Health (CU); 2018 [cited 2018 Jun 1]. 206 p. Available from: http://files.sld.cu/dne/files/2018/04/Anuario-Electronico-Espa%C3%B1ol-2017-ed-2018.pdf. Spanish.
- Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362(23):2155-65.
- Hamm CW, Bassand J-P, Agewall S, Bax J, Boersma E, Bueno H, et al. Guía de práctica clínica de la ESC para el manejo del síndrome coronario agudo en pacientes sin elevación persistente del segmento ST. Rev Esp Cardiol. 2012;65(2):173). Spanish.
- Ibánez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. Guía ESC 2017 sobre el tratamiento del infarto agudo de miocardio en pacientes con elevación del segmento ST. Rev Esp Cardiol. 2017 Dec;70(12):1082.e1-e61. Spanish.
- Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart Disease and Stroke Statistics-2018 Update: A report from the American Heart Association. Circulation. 2018 Mar 20;137(12):e67–492.
- Yu T, Tian C, Song J, He D, Sun Z, Sun Z. ACTION (acute coronary treatment and intervention outcomes network) registry-GWTG (get with the guidelines) risk score predicts long-term mortality in acute myocardial infarction. Oncotarget. 2017 Oct 11;8(60):102559–72.
- Fu R, Song C, Yang J, Wang Y, Li B, Xu H, et al. CAMI-NSTEMI Score- china acute myocardial infarction registry-derived novel tool to predict in-hospital death in non-ST segment elevation myocardial infarction patients. Circ J. 2018 Jun 25;82(7):1884–91.
- Dai S, Huang B, Zou Y, Guo J, Liu Z, Pi D, et al. The HEART score is useful to predict cardiovascular risks and reduces unnecessary cardiac imaging in low-risk patients with acute chest pain. Medicine (Baltimore). 2018 Jun;97(22):e10844.
- Song PS, Ryu DR, Kim MJ, Jeon KH, Choi RK, Park JS, et al. Risk scoring system to assess outcomes in patients treated with contemporary guideline-adherent optimal therapies after acute myocardial infarction. Korean Circ J. 2018 Jun;48(6):492–504.
- O’Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Catheter Cardiovasc Interv. 2013 Jul 1;82(1):E1–27.
- Brogan RA, Malkin CJ, Batin PD, Simms AD, McLenachan JM, Gale CP. Risk stratification for ST segment elevation myocardial infarction in the era of primary percutaneous coronary intervention. World J Cardiol. 2014 Aug 26;6(8):865–73.
- Rosselló X, Huo Y, Pocock S, Van de Werf F, Chin CT, Danchin N, et al. Global geographical variations in ST-segment elevation myocardial infarction management and post-discharge mortality. Int J Cardiol. 2017 Oct 15;245:27–34.
- Shimony A, Grandi SM, Pilote L, Joseph L, O’Loughlin J, Paradis G. Utilization of evidence-based therapy for acute coronary syndrome in high-income and low/middle-income countries. Am J Cardiol. 2014 Mar 1;113(5):793–7.
- Patel C, Burke JF, Patel H, Gupta P, Kowey PR, Antzelevitch C, et al. Is there a significant trans-mural gradient in repolarization time in the intact heart? Cellular basis of the T wave: a century of controversy. Circ Arrhythm Electrophysiol. 2009 Feb;2(1):80–8.
- Cruz Elizundia JM, Carmona Puerta R, Pérez Cabrera D. Significance and mechanisms of a prolonged QT interval in acute myocardial ischemia. CorSalud. 2013;5(1):130–2.
- Postema PG, Wilde AA. The measurement of the QT interval. Curr Cardiol Rev. 2014 Aug;10(3):287–94.
- Chávez González E. El intervalo QT, su origen e importancia del conocimiento de fórmulas para su medición en diferentes circunstancias clínicas. CorSalud. 2014 Jan–Mar;6(1):79–85. Spanish.
- Stankovic I, Putnikovic B, Janicijevic A, Jankovic M, Cvjetan R, Pavlovic S, et al. Myocardial mechanical and QTc dispersion for the detection of significant coronary artery disease. Eur Heart J Cardiovasc Imaging. 2015 Sep;16(9):1015–22.
- Akgumus A, Karaagac K, Peker T, Aydin O, Arican Ozluk O, Tenekecioglu E, et al. Can QT dispersion predict multi-vessel coronary artery disease in patients with acute coronary syndrome? Eur Res J. 2016 Mar 4;2(1):12–5.
- Helmy H, Abdel-Galeel A, Taha Kishk Y, Mohammed Sleem K. Correlation of corrected QT dispersion with the severity of coronary artery disease detected by SYNTAX score in non-dia betic patients with STEMI. Egypt Heart J. 2017 Jun;69(2):111–7.
- Van Dongen IM, Kolk MZH, Elias J, Meijborg VMF, Coronel R, de Bakker JMT, et al. The effect of revascularization of a chronic total coronary occlusion on electrocardiographic variables. A sub-study of the EXPLORE trial. J Electrocardiol. 2018 Sep–Oct;51(5):906–12.
- Moreno V, Marín F, Monmeneu JV, de la Morena G. Dispersión del intervalo QT y miocardio viable. Rev Esp Cardiol. 2009;62(4):461–3. Spanish.
- Acosta Martínez J, Berruezo A. Abordajes alternativos a la fracción de eyección en la estratificación de riesgo de arritmias ventriculares. Cardiocore. 2017 Jan–Mar;52(1):7–10. Spanish.
- Hetland M, Haugaa KH, Sarvari SI, Erikssen G, Kongsgaard E, Edvardsen T. A novel ECG-index for prediction of ventricular arrhythmias in patients after myocardial infarction. Ann Noninvasive Electrocardiol. 2014 Jul;19(4):330–7.
- Yu Z, Chen Z, Wu Y, Chen R, Li M, Chen X, et al. Electrocardiographic parameters effectively predict ventricular tachycardia/fibrillation in acute phase and abnormal cardiac function in chronic phase of ST-segment elevation myocardial infarction. J Cardiovasc Electrophysiol. 2018 May;29(5):756–66.
- Kenigsberg DN, Khanal S, Kowalski M, Krishnan SC. Prolongation of the QTc interval is seen uniformly during early transmural ischemia. J Am Coll Cardiol. 2007 Mar 27;49(12):1299–305.
- Gussak I, Antzelevitch C, editors. Electrical Diseases of the Heart. Vol. 2. 2nd ed. London: Springer; 2013.
- Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). Eur Heart J [Internet]. 2018 Aug 25 [cited 2018 Sep 20]. Available from: https://academic.oup.com/eurheartj/advance-article/doi/10.1093/eurheartj/ehy462/5079081
- Nowinski K, Jensen S, Lundahl G, Bergfeldt L. Changes in ventricular repolarization during percutaneous transluminal coronary angioplasty in humans assessed by QT interval, QT dispersion and T vector loop morphology. J Intern Med. 2000 Aug;248(2):126–36.
- Killip T 3rd, Kimball JT. Treatment of myocardial infarction in a coronary care unit. A two year experience with 250 patients. Am J Cardiol. 1967 Oct;20(4):457–64.
- Center for State Control of Medicines, Equipment and Medical Devices – CECMED (CU). Heberkinas A (Estreptoquinasa recombinante) Liofilizado para inyección (IV o IC). Titular del Registro Sanitario, Centro de Ingeniería Genética y Biotecnología (CIGB), Cuba. Número de Registro Sanitario: Heberkinasa® 1 500 000 UI: 1507. Fecha de Inscripción: Heberkinasa® 1 500 000 UI. 20 de marzo de 2000. Havana: Center for State Control of Medicines, Equipment and Medical Devices – CECMED (CU); 2000 Mar. Spanish.
- Bayés de Luna A. Bases de la electrocardiografía. De las variantes de la normalidad a los patrones diagnósticos (III): Isquemia, lesión y necrosis. Vol 3. Barcelona: ProusScience; 2007. 139 p. Spanish.
- Ahumada S, Restrepo G. Ecocardiografía en infarto agudo de miocardio. Rev Colomb Cardiol. 2014;21(3):133–98. Spanish.
- Center for State Control of Medicines, Equipment and Medical Devices – CECMED (CU). Registro de electrocardiógrafo digital de tres canales. Titular del Registro Sanitario, Instituto Central de Investigación Digital ICID. Fecha de Inscripción: 2008 febrero 28 [Internet]. 2008 Feb 28 [cited 2018 Sep 2]. Havana: Center for State Control of Medicines, Equipment and Medical Devices – CECMED (CU). 55 p. Available from: http://www.cecmed.cu/sites/default/files/adjuntos/registro_equipos_m/registro_em_2004_2007.pdf. Spanish.
- Ornek E, Duran M, Ornek D, Demirçelik BM, Murat S, Kurtul A, et al. The effect of thrombolytic therapy on QT dispersion in acute myocardial infarction and its role in the prediction of reperfusion arrhythmias. Nigerian J Clin Pract. 2014 Mar–Apr;17(2):183–7.
- World Medical Association. Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA [Internet]. 2013 Nov 27 [cited 2018 Jun 13];310(20):2191–4. Available from: https://jamanetwork.com/journals/jama/fullarticle/10.1001/jama.2013.281053
- Kosmala W, Przewlocka-Kosmala M, Halawa B. QT dispersion and myocardial viability in patients after acute myocardial infarction. Int J Cardiol. 2004 Apr;94(2–3):249–54.
- Jensen CJ, Lusebrink S, Wolf A, Schlosser T, Nassenstein K, Naber CK, et al. Reduction of QTD—A Novel Marker of Successful Reperfusion in NSTEMI. Pathophysiologic Insights by CMR. Int J Med Sci. 2015 May 3;12(5):378–86.
- George SK, Waly HM, Abdul Moteleb MT. Assessment of QT dispersion in patients with acute STEMI receiving thrombolytic versus those performing primary percutaneous coronary intervention (PCI) therapy. Med J Cairo Univ. 2015;83(1):1023–30.
- Eslami V, Safi M, Taherkhani M, Adibi A, Reza Movahed MR. Evaluation of QT, QT dispersion, and T-wave peak to end time changes after primary percutaneous coronary intervention in patients presenting with acute ST-elevation myocardial infarction. J Invasive Cardiol. 2013 May;25(5):232–4.
- Pekdemir M, Karaca I, Cevik Y, Yanturali S, Ilkay E. The diagnostic value of QT dispersion for acute coronary syndrome in patients presenting with chest pain and nondiagnostic initial electrocardiograms. Mt Sinai J Med. 2006 Sep;73(5):813–7.
- Machín Cabrera WJ, Pérez Chávez JL, Olivera Bacallao LO, Polanco Rodríguez F, Rodríguez Rueda JM, Fabelo Mora CJ. Dispersión del intervalo QT corregido en la evolución de pacientes con síndromes coronarios agudos. CorSalud [Internet]. 2011 [cited 2018 Sep 15];3(2):70–7. Available from: http://bvs.sld.cu/revistas/cors/sumario/2011/v3n2a11/intervalo.htm. Spanish.
- Ikonomidis I, Athanassopoulos G, Karatasakis G, Manolis AS, Marinou M, Economu A, et al. Dispersion of ventricular repolarization is determined by the presence of myocardial viability in patients with old myocardial infarction. A dobutamine stress echocardiography study. Eur Heart J. 2000 Mar;21(6):446–56.
- Lancellotti P, Bigle AR, Mipinda JB, Pierard LA. Significance of dobutamine-induced changes in QT dispersion early after acute myocardial infarction. Am J Cardiol. 2001 Nov 1;88(9):939–43.
- Chávez-González E, Rodríguez-González F, Machín-Cabreras W, González Ferrer V. Factores de riesgo asociados a mayor dispersión del intervalo QT corregido durante el infarto agudo de miocardio. Rev Fed Arg Cardiol. 2013;43(1):25–31. Spanish.
- Jiménez-Candil J, González Matas JM, Cruz González I, Hernández Hernández J, Martín A, Pabón P, et al. Pronóstico hospitalario del síndrome coronario agudo sin elevación del segmento ST determinado por una nueva escala de riesgo integrada por variables electrocardiográficas obtenidas al ingreso. Rev Esp Cardiol. 2010;63(7):851–5. Spanish.
- Rodríguez González F, Chávez González E, Machín Cabrera WJ, Reyes Hernández LM, González Ferrer V. Arritmias ventriculares y nuevo síndrome coronario agudo en pacientes con infarto y dispersión del intervalo QT prolongado. CorSalud [Internet]. 2013 [cited 2018 Jul 21];5(1):101–7. Available from: http://www.corsalud.sld.cu/sumario/2013/v5n1a13. Spanish.
THE AUTHORS
Ailed Elena Rodríguez-Jiménez (Corresponding author: ailedrj@infomed.sld.cu), physician with dual specialties in comprehensive general medicine and cardiology, and a master’s degree in satisfactory longevity. Associate professor, Camilo Cienfuegos General Provincial Hospital (HGPCC), Sancti Spíritus, Cuba.
Hugo Cruz-Inerarity, cardiologist. Instructor. HGPCC, Sancti Spíritus, Cuba.
Tessa Negrín-Valdés, physician with dual specialties in internal medicine and cardiology. Associate professor, HGPCC, Sancti Spíritus, Cuba.
Raikel Fardales-Rodríguez, cardiologist, HGPCC, Sancti Spíritus, Cuba.
Elibet Chávez-González, cardiologist with a doctorate in medical sciences. Associate professor, Ernesto Che Guevara Cardiology Center, Santa Clara, Cuba.
Submitted: October 01, 2018 Approved: June 22, 2019 Disclosures: None






