INTRODUCTION
In Central America, beginning in the 1990s, chronic kidney disease cases were reported unassociated with traditional risk factors (CKDnt), primarily affecting farming communities and male agricultural workers.[1] In 2000, it became apparent in Sri Lanka that a CKD of unknown etiology was affecting male rice farmers[2] and that its characteristics were similar to Balkan endemic nephropathy.[3] Recent reports of similar CKD cases in rural areas have come from Egypt[4] and India, where it is known as Udhanam endemic nephropathy.[5]
In Central America, a number of publications have chronicled the disease:
- García-Trabanino’s 2002 study of dialysis patients in El Salvador (1999–2000), found that cause of renal failure could not be identified in 67% of cases. This led to suspicion of a relationship with occupational exposure to insecticides or pesticides.[6]
- In 2003, Domínguez analyzed CKD prevalence and risk factors on the Pacific coast of southern Mexico, Guatemala, El Salvador and Honduras, finding an inverse association between proteinuria prevalence and municipal altitude. Among men with proteinuria living on the coast (altitude ≤200 m), 71% had no signs of hypertension (HT) or diabetes. Agricultural work and contact with pesticides were common to persons with CKD at all altitudes.[7]
- Research by Sanoff in León and Chinandega, Nicaragua (2003), reported endemic levels of CKD in young farmers, unrelated to diabetes or HT and associated with environmental and occupational exposures, working conditions, consumption of homemade liquor (lija) and drinking >5 L of water per day.[8]
- Prevalence of renal replacement therapy was 12.5
- cases/100,000 population in a study of dialysis and renal transplant patients in eight Salvadoran hospitals from August through November, 2003.[9]
- A 2005 study by García-Trabanino in Salvadoran farming communities detected proteinuria in 45.7% of coastal residents versus 12.9% of those living at high altitudes. Elevated blood glucose levels were also more common in coastal areas than in areas ≥500 m above sea level (25% vs. 8%, respectively). Proteinuria was not significantly related to agricultural work, pesticides or alcohol use.[10]
- Torres’ 2007 cross-sectional community study of CKD of unknown etiology in Nicaragua described prevalence of above-normal serum creatinine levels (definition: >1.2 mg/dL in men and >0.9 mg/dL in women) of 31% in male and 24% in female agricultural workers in a community at 100–300 m altitude.[11]
- According to the Latin American Society of Nephrology and Hypertension, in 2008, El Salvador reported 531 patients receiving renal replacement therapy per million population (pmp). Of these, 347 were on peritoneal dialysis, 121 on hemodialysis and 63 had received kidney transplants, figures above the mean for Central American countries of similar economic development levels.[12]
CKD in El Salvador is a serious public health problem and its epidemiology is not completely understood. It is the fifth leading cause of death nationwide in persons aged >18 years and the second cause of death in men. In 2009, prevalence of renal replacement therapy was 566 pmp.[13] According to the Ministry of Health’s 2011–2012 Annual Report, end-stage renal disease (CKD stages 3–5) was the third leading cause of hospital deaths in adults of both sexes (first for men and fifth for women), with an in-hospital case fatality rate of 12.6%.[14]
A study of farmers in five Salvadoran communities—two on the coast devoted to growing sugarcane, three at 500 m altitude with economies focused on services and non-sugarcane crops—found prevalence of chronic renal failure (CRF) (glomerular filtration rate, GFR <60 mL/min/1.73 m2 body surface area) of 18% on the coast, compared to 1% in communities at >500 m. Proteinuria was infrequent, or low grade, with no differences among communities. The study concluded that sugarcane cultivation in coastal areas was associated with decreased kidney function in patients studied, possibly related to strenuous work in hot environments with repeated fluid depletion.[15]
The Nefrolempa Study (2009) in rural communities of the Bajo Lempa region reported an all-stage CKD point prevalence in adults of 17.9%, higher in men (25.7% vs. 11.8% in women). CRF (stages 3–5) prevalence was 9.8%, higher in men (17% vs. 4.1% in women). Neither diabetes nor HT, nor any other primary renal disease accounted for the majority (54.7%) of cases.[16] Other population-based research in El Salvador found a CKD prevalence in adults of 15.4% (men 22.8%, women 9.5%) and CRF prevalence of 8.8% (men 15.9%, women 3.2%). CKD point prevalence observed varied from 13.3% to 21.1% (men 13.1%–29%, women 13.4%–21.5%). CRF point prevalence was 13.3%, higher in men (22.4% vs. 3% in women).[17]
Published overviews of CKDnt in the Central American region conclude that the disease has not been completely studied clinically or histopathologically.[1,18] This is also the case for El Salvador, where knowledge of its etiology, frequency and distribution in both the general population and in agricultural communities is incomplete, as is knowledge of its epidemiology, toxicoepidemiology, etiology and pathophysiology, anatomical pathology and clinical manifestations. This study’s objective was to characterize CKDnt’s clinical manifestations (including extrarenal) and pathophysiology in Salvadoran farming communities.
METHODS
A descriptive clinical study was conducted, involving 46 participants identified through population screening for CKD in 5018 persons in 11 agricultural communities in 4 regions of El Salvador; of them, 2388 were aged ≥18 years, of whom 431 had CKD, and of those, 134 were aged < 60 and in stages 2, 3a and 3b of the disease.[19] Of these patients, 60 gave informed consent to participate in the study and 46 met inclusion criteria.
Inclusion criteria were ages 18–59 years; CKDnt at stages 2, 3a, or 3b, or CKD at stages 3a or 3b with diabetes or HT and without proteinuria, reconfirmed prior to this study; normal fundoscopic exam; and no structural abnormalities on renal ultrasound. Exclusion criteria were CKD of known cause, HIV-positive, and any clinical or social condition that would prevent participation. Patients studied resided in four departments: Usulután (28 patients), San Miguel (8 patients), Ahuachapán (7 patients) and Chalatenango (3 patients).
The study period was March 3–April 20, 2013. Patients were hospitalized in San Juan de Dios National Hospital in San Miguel Department. A team of 70 researchers from 22 biomedical specialties participated from San Juan de Dios National Hospital and the National Health Institute, at the request of El Salvador’s Ministry of Health and in collaboration with PAHO and Cuba’s Ministry of Public Health.
Study variables Described in Table 1.
Data collection We approached the study from a biopsychosocial perspective. The study included analysis of social determinants, based on personal interviews conducted by a sociologist using a tailored questionnaire that covered structural and intermediate determinants in the life of each patient, the components of which are itemized in Table 1. Following a general, comprehensive clinical history, specialists conducted detailed examinations by organ and system pertinent to their respective specialties. A psychologist assessed each patient, including personality traits, behaviors and psychological response to their disease.
A specific questionnaire was used for nephrology assessment; tests included urine cytology, urine culture, and laboratory tests for markers of glomerular and tubular function, as well as renal damage. Renal, bladder, prostate and gynecological ultrasound were performed. Cardiovascular function was assessed by EKG, Doppler echocardiography, treadmill test and peripheral vascular Doppler ultrasound. The nervous system was assessed by physical examination, supplemented by ophthalmological examination (including fundoscopy, tonometry, and tests of visual acuity and fields). Audiometric testing was done. To assess the digestive system, serum enzymes were measured and hepatic and pancreatic ultrasound done. Other blood tests included coagulation profile, serotyping and viral serology. A dermatological examination was conducted.
Laboratory analyses were conducted by the San Juan de Dios National Hospital clinical laboratory, following the laboratory’s own standards and procedures manual and accepted international reference values.[19]
The following equipment and instruments were used: automated biochemistry analyzer (Siemens Dimension), immunoassay system (TriageArchitec Biosite), automated hematology analyzer (Sysmex XT-1800), osmometer (Advanced Instruments 3250 Single Sample), pH meter (Fisher Scientific accumet AB-150), CLINITEK Microalbumin 2 (Siemens), and blood gas analyzer (Nova Biomedical). All equipment was manufactured in the United States.
Table 1: Variables*

Table 1: Variables*

*Except as noted, all reference values from: Manual of Clinical Laboratory Standards, Procedures and Reference Values. San Juan de Dios National Hospital, San Miguel, El Salvador[19]
CH2O: free water clearance Cosm: osmolal clearance
E-Cosm: electrolyte osmolal clearance EF-Cosm: electrolyte-free osmolal clearance
GFR: glomerular filtration rate (per Modification of Diet in Renal Disease formula)[21]
Ethics The study was conducted at the request of the Ministry of Health of El Salvador (MINSAL, the Spanish acronym) and the research protocol was approved by the Salvadoran National Committee on Clinical Research of the Higher Council on Public Health. Written informed consent was obtained from all participants. The consent form described study objectives, benefits and risks, and provisions for confidentiality of the information obtained; and assured patients that they could withdraw at any time with no consequences for their medical care. Study participants received medical treatment as indicated by study findings.
Analysis LimeSurvey (an open-source online survey application installed on MINSAL’s server) was used to input and store information, and to design clinical history and data forms for access to all data—individual and aggregate—obtained. Data were filtered with the platform’s built-in tool and other software needed for specific purposes. Design included procedures to validate data before entry into the database, inclusion of imaging and other binary elements, and geocoding of each participant’s home and workplace. Information was entered into a database and exported to SPSS for calculation of frequencies (point estimates and 95% confidence intervals) of study variables.
RESULTS
Age and sex distribution Patient age group distribution was as follows: 18–29 years, 2 patients (4.3%) (one diagnosed at age 16 years); 30–39 years, 11 (23.9%); 40–49 years, 14 (30.4%); and 50–59 years, 19 (41.3%). Mean age: 45.4 (95% CI 42.7–48.1). Of the 46 patients studied, 36 (78.3%) were men and 10 (21.7%) were women (Table 2).
Table 2: Risk factor prevalence in Salvadoran CKD patients (n = 46)
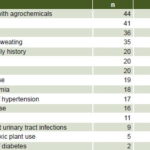
CKD: chronic kidney disease
Social determinants The sociological study found that patients’ community habitat is characterized by poverty, homes in poor condition, poor quality drinking water, low educational level, poor diet, inadequate health services, and inadequate domestic electricity. In addition, farmers’ working conditions are characterized by indiscriminate use of agrochemicals (combining several at once, some prohibited, without protection for the farmer and with consequent environmental contamination), as well as long hours of intense outdoor physical activity, and profuse sweating in the absence of adequate hydration.
Psychological characterization The assessment of personality traits and mental health indicators showed that the psychological sphere and behavior patterns of these patients were characterized by anxiety, depression, bargaining phase subsequent to denial of their illness, domestic violence, alcohol consumption and fear of dying.
General symptoms Arthralgia was reported in 54.3% (25) of cases (and from the earliest stages); asthenia in 52.2% (24); decreased libido in 47.8% (22); cramps in 45.7% (21); and fainting in 30.4% (14).
Renal symptoms Disorders of micturition were the most common symptoms: nycturia in 30 patients (65.2%); dysuria in 18 (39.1%); post-void dribbling in 15 (32.6%); urinary hesitancy in 9 (19.6%); and foamy urine in 29 (63%). All symptoms were seen as early as stage 2 and tended to increase as the disease advanced. Some, including thin stream, urinary hesitancy, and dysuria were more evident from the start.
Body mass index Underweight, 0%; normal weight, 21 (45.7%); overweight, 22 (47.8%); and obese, 3 (6.5%).
Genitourinary system The most common risk factors reported were contact with agrochemicals in 44 patients (95.7%), farming 41 (89.1%), male sex 36 (78.3%) and profuse sweating during the workday 35 (76%). Only two reported being diabetic. See Table 2 for prevalence of other risk factors.
Markers of renal damage Urine sediment showed no significant abnormalities or dysmorphic erythrocytes. Urine cultures were negative. Albumin-to-creatinine ratio was >300 mg/g in 37 patients (80.4%), 30–300 mg/g in 7 (15.2%), and <30 in 2 (4.3%). The ratio remained similar from early stages of the disease. Proteinuria >1g was present in 1 patient (2.2%); β2 microglobulin was elevated in 36 (78.2%); and NGAL was increased in 12 patients (26.1%).
Patient distribution by CKD stage Distribution was as follows: stage 2, 15 patients (32.6%); stage 3a, 11 (23.9%); stage 3b, 20 (43.5%).
Imaging Renal ultrasound showed the traditional CKD pattern with a prevalence of increased echogenicity in 44 patients (95.7%), decreased corticomedullary ratio in 38 (82.6%), and irregular margins in 35 (76.1%). Renal Doppler ultrasound showed that 44 patients (95.7%) had normal blood flow in renal arteries, segmental arteries and renal parenchyma. Bladder ultrasound showed wall thickening in 2 patients (4.3%), increased residual volume in 5 (10.9%), and nonmalignant intravesical lesions in 4 (8.7%). Prostate ultrasound found normal echogenicity in all patients; 10 (27.8%) had increased prostate volume; and no malignant lesions were found. Gynecological ultrasounds were normal.
Renal function tests Polyuria was present in 24 patients (52.2%), predominantly electrolyte polyuria (20, 43.5%); a single patient (2.2%) exhibited electrolyte-free solute polyuria, and 3 (6.5%) mixed polyuria. All patients had hypermagnesuria, 23 (50%) had hyperphosphaturia, 21 (45.7%) hypernatriuria, 11 (23.9%) hyperkaluria, and 8 (17.4%) hypercalciuria. Fractional excretion of magnesium was increased in all patients and of sodium in 18 (39.1%). Serum electrolytes reflected the excess exertion observed in urine (Table 3).
Table 3: Blood and urine electrolytes in Salvadoran CKD patients (n = 46)
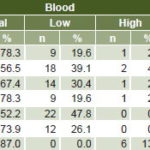
Blood osmolality was normal in all patients. Urine osmolality was normal in 35 (76.1%); 5 (10.9%) presented urine hypo-osmolality and 6 (13%) had hyperosmolal urine. The predominant acid-base balance disorder was metabolic alkalosis in 21 patients (45.7%). Metabolic acidosis was present in only 2 patients (4.3%). Acid-base and electrolyte disorders in urine and blood began to appear in stage 2 CKD.
Cardiovascular system In 30 patients (65.2%), heart rate was 60–79, in 15 it was 80–89 (32.6%), and 1 (2.2%) was bradycardic. On admission, 20 patients (43.5%) were normotensive, 16 (34.8%) had prehypertension, 7 (15.2%) had stage-1 HT, and only 3 (6.5%) had stage-2 HT. Mean systolic blood pressure was 116.5, (95% CI 111.1–121.9); mean diastolic blood pressure, 74.1 (95% CI 70.7–77.5); and mean arterial pressure, 88.2 (95% CI 83.7–92.7). EKG was normal in 45 patients (97.8%). On the cardiac stress test, 39 patients (84.8%) presented excellent physical capacity and 6 (13%) good capacity. Pressor response was abnormal in 5 cases (10.9%), Echocardiogram (n = 45) was normal in 23 cases (51.1%) (1 patient was unable to complete ultrasound testing); in 22 patients (48.9%), mild diastolic dysfunction was detected; concentric left ventricular hypertrophy in 7; and contractile dysfunction in 1 patient. Peripheral artery Doppler ultrasound found few abnormalities of the carotid and aortoiliac arteries, but two thirds of patients (30/45) had tibial artery abnormalities and 37.8% (17/45) of the femoral arteries. The most common tibial artery lesion was wall irregularity in 22 patients (48.9%), most common in stage 3b (11/20, 55%), but seen even in stage 2 (6/14, 42.9%) (Table 4).
Nervous system Neurological abnormalities were seen in 21 patients (45.7%). Three (6.5%) had Babinski sign and myoclonus. Tendon reflex abnormalities were seen as early as stage 2 (Table 5). Sensorineural hearing loss was evident in 35 cases (76.1%). Visual acuity showed typical age-related changes; fundoscopic, intraocular pressure and visual fields tests were normal.
Digestive system All patients had normal liver enzymes and negative viral serology. Hepatic ultrasound found fatty liver in 43 patients (93.5%). Pancreatic amylase was normal in 29 patients (63%), elevated in 15 (32.6%), and low in 1 patient (2.2%).
Hemopoietic system Patients’ mean hemoglobin was 14 g/dL (95% CI 13.5–14.4). Only 6 had below-normal hemoglobin values. No coagulation abnormalities were found.
Lipid metabolism Hypercholesterolemia was present in 24 patients (52.2%), with a mean serum cholesterol of 213.1 mg/dL (95% CI 199.0–221.2); hypertriglyceridemia in 24 (52.2%), mean 192.0 (95% CI 153.4–230.6); elevated LDL in 38 (82.6%), mean 137.7 (95% CI 125.4–150.0); and HDL normal in 25 (54.3%) and elevated in 9 (19.6%), mean 50.7 (95% CI 43.5–57.9).
Carbohydrate metabolism Glucose was normal in 43 patients (93.5%), high in 2 (4.3%) and low in 1 (2.2%); mean fasting blood glucose was 92.2 mg/dL (95% CI 88.3–96.1). Glycosylated hemoglobin was normal in 39 patients (84.8%) and high in 7 (15.2%); mean fasting blood glucose was 5.8% (95% CI 5.7–5.9).
Table 4: Peripheral arterial Doppler ultrasound in Salvadoran CKD patients (n = 45a)
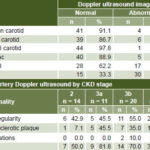
a one patient unable to complete testing / b patients could have more than one abnormality
Table 5: Neurological abnormalities in Salvadoran CKD patients (n = 46)
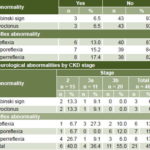
Respiratory system Spirometry and chest x-ray were normal in all patients.
Skin No dermatological lesions were detected specific to damage from metal exposures.
DISCUSSION
The fact that patients with CKDnt come from farming communities, and that more are male farmers, with fewer women and adolescents, requires analysis of contextual factors related to participants’ place of work and residence. Three general conditions that affect these patients were identified: poverty, with all its repercussions; unhealthy working conditions; and a contaminated environment. These elements link the disease to deep social roots. These families’ living conditions, coupled with the impotence of watching the disease’s progression towards death of loved ones, with no solution in sight, have plunged them into a state of grief.
The relatively high prevalence of NSAID use could be related to the high proportion of patients reporting joint pain. This in turn could be due to their strenuous work, and more research is needed to determine whether a toxic component may also contribute to pain. The cramping and fainting described could be the result of hyponatremia, seen in almost half the patients.
Lower urinary tract symptoms reported by patients, evident from early stages of the disease, mimic lower urinary tract obstructive syndrome, but most patients had neither obstruction nor urinary infection. Similar symptoms, without urinary infection, have been reported in Nicaraguan farmers, a condition called chistata.[18] Neurotoxic bladder irritation is one possible cause. Foamy urine is the manifestation of macroalbuminuria, detected in most study participants.
The fact that few patients had a history of traditional CKD risk factors—diabetes, HT and obesity—makes it unlikely that CKDnt is caused by the same factors that drive the global CKD epidemic. The main risk factors identified were nontraditional ones: contact with agrochemicals, agricultural work, male sex, family history of CKDnt, history of malaria, profuse sweating and use of NSAIDs. The predominance of male farmers suggests work-related risk factors are important, but the appearance of the disease in women and adolescents implies that there are additional risk factors to which the general population is also exposed. This raises the possibility of chronic low-level exposure to environmental toxins from proximity to agricultural activities.
Normal kidney function increases risk of renal damage from environmental toxins due to the high volume of renal blood flow, since large quantities of toxic substances can pass through the kidneys. The kidney’s capacity to concentrate substances through filtration, reabsorption and secretion can increase the toxicity of agents that in low concentrations would not lead to renal damage. CKD may be manifested in chronic tubular defects, as has been seen in chronic poisoning from cadmium, lead and other agents. Furthermore, patients with previous renal damage are vulnerable to toxicity of substances that normally are excreted in the urine.[24] Thus, further studies are needed of environmental contamination and measurement of toxins in biological fluids.
Research in Sri Lanka found elevated urinary arsenic concentrations in patients with CKD of unknown etiology and detected high cadmium concentrations in well water where patients lived. The authors considered pesticides a possible source of environmental contamination by these metals.[25,26] Moderately high levels of urinary and blood cadmium were found to be associated with a higher proportion of albuminuria and CKD in NHANES study participants in the USA.[27] Almost all our cases had contact with pesticides.
The markers of renal damage observed made clear—because of the absence of proteinuria >1g—that this is not a proteinuric glomerular disease. Rather, tubulointerstitial damage is suggested by biomarkers of tubular damage, such as elevated urinary β2 microglobulin levels. It has been posited that elevated β2 microglobulin, NGAL and NAG may be associated with damaged proximal renal tubules.[28,29] A study of eight Salvadoran farmers with CKDnt found elevated β2 microglobulin and NAG, and tubulointerstitial damage was corroborated by renal biopsy.[30] It has long been known that chronic exposure to high doses of cadmium is associated with decreased renal tubular reabsorption of β2 microglobulin, as shown in an early study of work-related renal disease.[31]
Renal ultrasound and Doppler ultrasound ruled out other traditional causes of CKD such as polycystic disease, vascular nephropathy and obstructive nephropathy. In most cases, no lower urinary obstruction was detected in bladder, prostate and gynecological ultrasounds.
Electrolyte loss in urine—primarily magnesium, phosphorus, sodium and potassium—beginning in early stages of the disease, signifies primarily tubular damage as the initial site of renal damage, which once more points to chronic tubulointerstitial nephropathy. The proximal tubule reabsorbs 60% of electrolytes, which is why this must be the segment most involved. This electrolyte loss explains the electrolyte polyuria and low concentrations of some electrolytes in blood, as well as the symptoms of cramping and fainting. The absence of acidosis could indicate a relative conservation of the distal segment of the nephron, with bicarbonate reabsorption and hydrogen ion excretion.[32]
López-Marín’s histopathological characterization of renal biopsies from these same patients corroborated that chronic tubulointerstitial nephropathy was the initial damage.[33] Histopathology studies in Sri Lanka had similar results.[34] Wijkström’s study of eight Salvadoran patients identified damage to both glomerular and tubulointerstitial compartments, but nearly all were in advanced stages of CKD,[30] when all tissue compartments are typically compromised.[19]
However, this form of chronic tubulointerstitial nephropathy in Salvadoran farming communities has extrarenal manifestations not attributable to renal disease progression, which suggests factors that could damage the kidney and other organs at the same time.
The main complications of traditional CKD from its early stages are cardiovascular.[35,36] However, in our patients, only a small percentage were hypertensive, they had relatively low heart rates, and most had normal findings on EKG, stress test and cardiac Doppler ultrasound. The fact that the study patients were relatively young and members of populations with very low prevalence of HT, diabetes, obesity and smoking, suggests that vascular damage from these factors is minimal and that the vascular damage observed is more likely caused by their CKD rather than the converse. The protective effect of exercise in physically demanding jobs is evident in the almost athletic performance of the patients on the treadmill test. These facts could make CKDnt an interesting clinical model for studying the cardiovascular impact of CKD isolated from other vascular risk factors.
Traditionally, CKD patients have a high prevalence of peripheral vascular disease from the confluence of two pathological abnormalities—atherosclerosis and arteriosclerosis—that are more frequent in patients with obesity, diabetes and HT, the peripheral vascular damage progressing with CKD evolution. It has been reported that frequency of atherosclerotic plaques in carotid arteries is four time greater in CKD patients than in controls.[37,38] In contrast, our patients’ carotids were relatively unharmed, with the main vascular damage occurring in the tibial arteries. Atherosclerosis in all upper arteries was rare, becoming more evident in the lower body and peaking in the tibial arteries. One hypothesis for this selective damage to tibial arteries could be their greater contact with toxic substances on the job. Farmers’ legs, sometimes bare, are the parts most exposed to agrochemicals from spraying, which is done using backpack applicators at high ambient temperatures, with consequent vasodilation and opening of skin pores. A Japanese study of patients with chronic arsenic exposure, showed that this metal produced endothelial dysfunction from inhibition of endothelial nitric oxide synthase enzyme and decreased nitric oxide production, associated with overproduction of reactive oxygen species, both inductive mechanisms for vascular damage.[39]
From early stages of the disease, symptoms of anterior motor neuron damage—reflex disorders—were detected, as well as Babinski sign and myoclonus. The sensorineural hearing loss found did not correspond to deafness related to hereditary nephropathy (which shows predominantly glomerular damage and is accompanied by proteinuria). Uremic neurotoxicity does not explain the neurological symptoms we detected in early CKD stages. Heavy metal and pesticide exposure have been associated with nervous system diseases (Parkinson, Alzheimer, amyotrophic lateral sclerosis), dopaminergic system impairment, impaired nerve conduction velocity, diminished reflexes, irritability, memory loss and other diseases.[40,41]
Almost all patients had fatty liver with normal enzymes; this was associated with the risk factors of dyslipidemia and/or alcohol consumption. It must be kept in mind that the liver is the main organ for metabolism and removal of toxins. Dyslipidemia was present in the majority of cases, possibly influenced by diets high in fats and calories, associated with metabolic disorders typical of CKD.[42] Paradoxically, HDL was normal or high in most patients, which could reflect the protective effect of their physical activity.
People in farming communities are subjected to the same traditional CKD risk factors as the rest of the world’s population. However, the minimal presence of traditional risk factors in study patients points to environmental and occupational factors that could act synergistically to exacerbate a predominant one. Agricultural workers are also exposed to many toxic substances contained in dozens of agrochemicals, many of them prohibited, yet used in large quantities and mixed together without protection. In addition, these farmers carry out intense physical activity during long hours in high temperatures, without adequate hydration.[15,43] It is noteworthy that, although the disease occurs primarily in male farmers, it also affects women and adolescents, who do not necessarily work in the fields.
The clinical picture of CKDnt in this study is consistent with the hypothesis of environmental toxic agents (heavy metals and chemicals) from natural sources or from human activity as the pathogenetic trigger. Such toxins could be present in air, soil, water and food; subject to transformation by weather, topography and land use; and transported by air, water, clothing and food. Occupation, behaviors and drinking water quality could facilitate chronic exposure through inhalation, ingestion and/or skin contact.
Different levels of exposure are possible: a consistently high level over time from multiple acute exposures becoming chronic, primarily affecting farmers; and chronic low-level exposure affecting the general population, as well as farmers. In both cases, there could be interaction with genetic susceptibility.
In addition to chronic circulation in the blood of toxins eliminated through the kidneys, in agricultural fields with high temperatures, these toxins also concentrate in the renal medulla under the effects of dehydration from profuse sweating and low fluid intake.[15,18,24,44,45]
Besides this cascade of events, other influences are undoubtedly at work, such as social conditions—poverty paramount among them—that increase the likelihood of renal damage from low birth weight due to maternal malnutrition, infectious diseases (such as malaria), diabetes, HT, alcohol consumption, NSAID use and other factors.[21]
Chief among the study’s limitations is its small sample size, insufficient for estimating extent and significance of associations among such a large number of variables. Furthermore, we were unable to measure toxins in biological fluids. On the other hand, this is the largest clinical study of CKDnt in the Americas to date, with the greatest multidisciplinary involvement and the most thorough treatment of clinical and pathophysiological aspects, and including study of women and adolescents. Also, since over half of study patients were in CKD stages 2 and 3a, it permitted analysis of disease course from early stages.
CONCLUSIONS
CKDnt in Salvadoran farming communities is associated with social and working conditions and behaves like a chronic tubulointerstitial nephropathy. It has extrarenal manifestations not attributable to the progression of renal disease, suggesting that the kidney damage is a component of a more systemic process. This is compatible with the hypothesis of multifactorial etiopathogenesis with environmental nephrotoxic agents at its core. Environmental and biological toxicology studies should further explore the working conditions of farmers and the behavior of this disease in women, children and adolescents in these communities.
ACKNOWLEDGMENTS
The authors thank the following collaborators: José M. Pacheco Paz, José R. Centeno Paz, Elsy Guadalupe Brizuela, Alfonsina Chicas, Reyna Jovel, Nelly Alvarado Ascencio, Carlos J. Martín Pérez, Rigoberto Machuca Girón, Manuel A. Zúñiga Fuentes, Nelson E. García Alvarez, Guadalupe M. Imbers de Rubio, María E. Melgar de Reyes, Henry N. Laínez Lazo, José R. Hernández Franco, Yesenia E. Guevara, Magdalena I. Zelaya Rivera.









