INTRODUCTION
Tuberculosis (TB) is one of the main causes of morbidity and mortality in many countries and is considered a priority global health problem. Although it is a curable disease, every year some nine million people develop active disease and two million die from it.[1,2] WHO has coordinated multiple efforts towards global TB control. A goal of the Global Plan to Stop TB is to reduce TB deaths by half from 1990 to 2015.[1]
Monitoring TB chemotherapy outcomes is essential for TB elimination.[3,4] Even under treatment, >10% of patients can die when adherence is low or when rates of HIV infection and multidrug-resistant TB (MDR-TB) are high.[5,6] Studies of adverse outcomes of TB chemotherapy have associated them with advanced age, alcoholism, HIV infection (more common among men), previous treatment with TB drugs and the rising rate of extrapulmonary forms of TB.[7,8]
Cuba has 11.2 million inhabitants and a population density of 102/km2.[9] The first National Tuberculosis Control Program (PNCT) was established in 1963, and since, TB has been a notifiable disease.[10] Cuba was an early adopter of the global Directly Observed Treatment Short Course (DOTS) strategy, implementing in 1971 a strategy based on intensive contact tracing and strictly controlled ambulatory treatment, and using standard treatment protocols and case definitions based on WHO recommendations.[5,11,12] Cuba has low TB incidence for a middle-income country,[13] an indicator of PNCT’s impact, and is on WHO’s list of countries making progress towards TB elimination.[14]
Cuba has one of the lowest TB incidence rates in Latin America,[15] at 6.1/100,000 population in 2012.[16] Two studies from 2009–2010 in western[17] and central[18] Cuba found pulmonary TB the most common form and death the most important adverse outcome of TB chemotherapy. In 2014, the TB mortality rate was 0.2/100,000 population.[19] There are no available publications with in-depth evaluations of the determinants of TB case fatality in Cuba.
The purpose of this study was to assess survival patterns and predictors of death in Cuban patients with pulmonary TB.
METHODS
Population and study design This was a retrospective cohort study in Cuba with all notified cases of pulmonary TB in which patients started chemotherapy between January 1, 2009 and December 31, 2010. The censoring date was September 17, 2011. We studied factors to which patients were exposed before starting chemotherapy, to assess possible associations with case fatality.
Data collectionWe reviewed the database and notifiable disease records of the National Medical Records and Health Statistics Bureau of the Ministry of Public Health (MINSAP). The database contains information from death certificates (primary data), which are filled out by physicians. Epidemiologic histories of notified TB cases were also reviewed, as were patient treatment records.
Data on drug culture and sensitivity test results were obtained from the National Reference and Research Laboratory on Tuberculosis and Mycobacteria of the Pedro Kourí Tropical Medicine Institute (IPK).
Definitions Patient age (at diagnosis) was analyzed as a quantitative variable and dichotomized based on the median.
Patients were classified as follows:
- New case: patient who had never received TB drugs or was treated for less than 30 days and was not previously reported as a TB case[10]
- Retreated: patient retreated after chemotherapy failure, treatment interruption, or recurrence (patient diagnosed bacteriologically with TB after being declared cured or receiving complete cycle of chemotherapy)[10]
- Deceased: patient who died (for any reason) during chemotherapy or who completed treatment and died during study period.[10] In both cases, TB appears on death certificate as a cause of death.
Patients whose sputum smear microscopy revealed acid-fast bacilli (AFB) were classified as having AFB+ pulmonary TB; if bacteriological sputum examination was negative, they were deemed to have AFB– pulmonary TB.[10]
Patients who were incarcerated during the study or who reported having been so at any point in their lives were defined as having a history of incarceration.
TB diagnosis was made following PNCT protocols in various health settings: family doctor-and-nurse offices, polyclinics (community-based multispecialty clinics at the primary level, to which family doctor-and-nurse offices report) or hospitals. Prisoners with TB were diagnosed in penitentiary infirmaries.[10]
Drug sensitivity tests on isolated strains of all TB cases identified two groups for comparison: resistant and sensitive. A case was considered resistant when the patient had a strain resistant to at least one TB drug.[10]
Analysis A descriptive analysis was conducted using the chi-square and Fisher exact tests to compare categorical variables, with a statistical significance level of 0.05.
For survival analysis, censoring was established by analyzing the number of days from start of chemotherapy to the censoring date or exit date when the patient was released from the cohort (cured; or treatment completed, failed, or abandoned). The Kaplan-Meier method was used to estimate survival. The log-rank test was used to compare survival curves of the different categories of a single variable. The Cox univariate model was also used and a multivariate model was adjusted to calculate risk of death, and the Wald test was applied; the best model was selected and risks considered significant (by hazard ratio, HR) were reported, with 95% confidence intervals.
The assumption of risk proportionality was verified graphically and by goodness of fit with the Grambsch and Therneau test based on analysis of residuals.[20] The programs SPSS Statistics 19 and R 3.3.0 were used.
Ethics The study was approved by IPK’s Scientific Council and Ethics Committee and by PNCT and MINSAP coordinators, in compliance with ethical requirements for studies using administrative data. Data management procedures ensured patient confidentiality.
RESULTS
From January 2009 to December 2010 in Cuba, 1339 cases of pulmonary TB (87.6% of all notified TB cases) were notified and chemotherapy was initiated. Of these, 77.4% were men; mean age was 48 years (SD 18; median 47, interquartile range 36–62). Most (92.1%, 1233) were new cases and 72.6% (972) were AFB+; 5.7% (76) were seropositive for HIV; and 4.2% (56) had diabetes. History of alcoholism was reported in 16.6% (222) cases. History of incarceration was reported in 18.1% (243). Of 257 sputum sensitivity tests performed, 7.4% (19) found resistance to at least one TB drug (Table 1).
Case fatality was 7% (94/1339), significantly higher among men, patients aged ≥48 years at time of diagnosis, those coinfected with HIV and those without history of incarceration (Table 1).
Average length of observation of pulmonary TB patients was 208 days (SD 58). Maximum length of observation was 487 days. Average length of observation in the 94 cases of death within the study period was 97 days (SD 110, median 56, interquartile range 22–131); 55.3% of deaths occurred during the first 60 days of treatment (Table 2). Case fatality in the first 60 days of chemotherapy was 3.9% (95% CI 2.8–4.9); case fatality later in the observation period was 3.3% (95% CI 2.3–4.3, p = 0.45).
Table 1: Pulmonary tuberculosis and deaths, Cuba, 2009–2010

AFB: acid-fast bacillus TB: tuberculosis achi-square test bFisher exact test
Survival analysis covered the observation period of the 94 pulmonary TB patients who died and 1245 patients with censored data. Table 3 presents Cox univariate analysis for mortality prediction. We found significant differences in survival by sex, age, TB/HIV coinfection, and presence/absence of a history of incarceration (Figure 1). Case category (new vs. re-treated), history of alcoholism, place of diagnosis and resistance to ≥1 TB drug had no significant influence on survival rates.
In multivariate analysis, risk of dying for men with pulmonary TB was almost twice as high as for women (HR 1.87, 95% CI 1.02–3.45). Age ≥48 years at diagnosis almost quadrupled risk of death (HR 3.93, 95% CI 2.41–6.40). Risk of death in patients with TB/HIV coinfection was six times that of patients without HIV (HR 6.25, 95% CI 3.46–11.31). The remaining variables were excluded from the model as they were not significant.
Table 2: Time between start of TB chemotherapy and death in patients who died of pulmonary TB, Cuba, 2009–2010
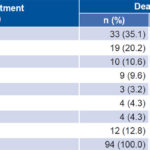
TB: tuberculosis
Table 3: Mortality prediction in Cox univariate analysis
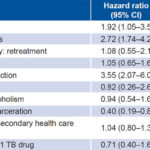
AFB: acid free bacillus CI: confidence interval PTB: pulmonary tuberculosis
TB: tuberculosis
DISCUSSION
Countrywide application of standardized techniques of TB diagnosis, record-keeping and case notification (as well as death certification) ensures data validity and reliability. This study was the first to introduce survival analysis in a population-based study of pulmonary TB in Cuba.
The finding that pulmonary TB affected mostly adult men in their economically active years is consistent with global patterns.[6,8] According to WHO, TB is more common among men and affects mainly working-age adults (approximately two thirds of people with TB are aged 15–59 years).[2,4] Poorer survival observed in patients aged ≥48 years is also in line with global patterns.[21,22] Studies in Mexico and Taiwan also reported lower survival in older groups: aged >45 years in Chiapas, Mexico[23] and aged >65 years in Taipei, Taiwan.[8]
Poorer survival in men than in women is consistent with studies in Brazil,[24] India [25] Israel,[26] and in WHO’s global TB reporting.[2] In Cuba, crude all-cause mortality is also higher for men (8.6/1000 vs. 7.3/1000 for women in 2012).[16] A 2010 study on social determinants of mortality in Cuba found that men were more exposed to risk factors (such as smoking and occupational hazards) and hypothesized that the so-called “masculine role” encourages risk-taking behavior.[27] Other authors have found no differences in men’s and women’s TB mortality and survival: Pardeshino in India in 2009[28] and Kim in Korea.[29] Neither study, however, was population based; the Indian study was conducted in a TB treatment center in the district of Yavatmal[28] and the Korean study was of 960 patients at Yonsei University Hospital.[29]
Diabetes mellitus is recognized as a risk factor for active TB,[30,31] but we found few cases of diabetes, and no association with case fatality, possibly due to small sample size. Cuba’s absence of economic barriers to primary care and diabetes treatment may also have contributed.[32]
Despite its low prevalence in the study cohort, TB/HIV coinfection was positively associated with case fatality. WHO statistics for 2011 indicated that 13% of TB cases worldwide had TB/HIV and 430,000 died.[2] HIV has been shown to be an important predictor of mortality for TB patients in countries such as Estonia,[33] Brazil,[34] Zambia and Malawi.[35] In turn, tuberculosis is a predictor of mortality in patients with HIV.[36] Maruza reported that HAART improved survival in HIV patients coinfected with TB, and that mortality increased with duration of HAART and compromised immune status.[34]
AFB+ pulmonary TB, the most common form of TB found in the study, is also the most common in other Cuban studies and globally.[2] Llanes reported that in Cuba from 1992 to 2002 AFB+ pulmonary TB was the most widespread, with 69.5% of total pulmonary TB.[37] In Europe, Jordan and Davies reviewed articles on TB chemotherapy outcomes published in 2009 and reported that 60% of pulmonary TB cases in the region were AFB+. In Southeast Asia, however, it was 37.4%, and in Tanzania, Africa, it was 41%.[38] This could be because these areas have high prevalence of TB/HIV coinfection and more cases of AFB- and extrapulmonary TB.[39,40]
Figure 1: Survival analysis by sex, age, TB/HIV coinfection and history of incarceration

Alcoholism is known to increase susceptibility to M. tuberculosis infection through immune system depression (it also affects TB disease course through pharmacokinetic and other mechanisms).[41] In the United States, Mitruka studied 24 TB outbreaks occurring between 2002 and 2008 and found that in 19, at least 40% of patients were alcoholic.[42] Rehm estimated that 10% of TB cases worldwide are attributable to alcohol.[41] Although 16% of pulmonary TB patients we studied were alcoholic, there was no association with case fatality, in contrast to the findings of Rehm’s systematic review.[41]
Although we found that numbers of pulmonary TB cases and deaths were both lower in persons with history of incarceration than in the general population, it has been reported internationally that TB prevalence among prisoners can be 50 times higher than in the overall population.[43–46] Vinkeles Melchers reviewed 52 scientific articles on TB control in prisons and found descriptions of shortcomings in TB control program implementation in such facilities.[47] Perhaps Cuba has succeeded in avoiding these problems because it adheres to WHO recommendation for active TB case-finding in vulnerable groups, including prisoners and exprisoners.[1,3] Gonzalez-Ochoa’s study in Las Tunas Province found that combining passive TB case-finding in health services with active case-finding in vulnerable communities (where exprisoners lived) was five times more effective than passive case-finding alone.
He emphasized the importance of such a combined strategy in areas of low TB incidence.[48]
While our study did not find an association between M. tuberculosis drug resistance and case fatality, other studies have done so.[2] In Estonia, Kliiman reported that patients with MDR pulmonary TB had a greater than eightfold risk of death (HR 8.56; 95% CI:1.81–40.4).[33] In Singapore, Low also reported a significant risk of death for MDR patients.[49] It should be borne in mind that MDR TB is relatively infrequent in Cuba, reflecting the high priority given to directly observed treatment.[50]
The higher relative frequency of deaths within the first two months of TB chemotherapy may be attributable to delayed diagnosis and treatment. Treatment delay can affect clinical severity[51] and increase risk of death and community transmission.[52] Evaluations of case detection procedures in several Cuban municipalities documented delays “attributable to the patient” (delays between respiratory symptom onset and first medical appointment),[53,54] which affected promptness of diagnosis and treatment. Such findings reinforce the importance of active case-finding in vulnerable groups.
A limiting factor in the study was lack of data on a range of risk factors that might affect survival: nutritional status, presence of other chronic diseases, smoking, HAART adherence, immune status and other factors not available in records consulted. Nevertheless, the population-based nature of the study makes it useful as a basis for future research aimed at reducing mortality and eliminating TB in Cuba.
CONCLUSIONS
The higher relative frequency of deaths early in TB treatment course may be related to diagnostic and/or treatment delays. Older age at diagnosis, male sex and TB/HIV coinfection increase risk of death.







