INTRODUCTION
The term cerebrovascular disease (CVD) includes all disorders in which an area of the brain is temporarily or permanently affected by ischemia or hemorrhage, with one or more of the cerebral blood vessels affected by disease. Stroke is a generic term referring to a group of disorders that include cerebral infarction, cerebral hemorrhage, and subarachnoid hemorrhage, and that describes the abrupt and sudden nature of onset.[1]
Stroke is the third leading cause of death in the world,[1,2] with highest mortality in low- and middle-income countries. According to the World Health Organization (WHO), about 5.71 million people died from stroke in 2004,[3] and it is estimated that this number will climb to 6.3 million in 2015 and 7.8 million in 2030.[4] In 2001, 85.5% of the world’s stroke deaths occurred in developing countries, where loss of disability-adjusted life years (DALYs) was seven times higher than in developed countries.[5] In the past 20 years, in developed countries, there has been a 29% decline in the incidence of all types of stroke, especially in women, and a 25% reduction in mortality, except for hemorrhagic stroke.[2,5]
In Cuba, stroke is also the third cause of death and fourth cause of DALYs lost; in 2009, crude mortality from stroke was 83.7 per 100,000 population, and the adjusted rate 46.7 per 100,000 population.[6]
According to WHO and the Pan American Health Organization (PAHO), in 2000, stroke was the first or second leading cause of death in 25 Latin American and Caribbean countries, and in 2002, it caused 272,000 deaths in 27 countries of the region.[4]
The literature is sparse on epidemiological studies of stroke in Latin America and the Caribbean. Published papers report lower prevalence and different patterns than those observed in developed countries. Protective factors, including ethnic origin, dietary or lifestyle differences, or increased mortality in the acute stroke phase are cited to explain the lower rates reported.[7,8]
Developing countries are still undergoing a rapid, unparalleled demographic transition in which chronic noncommunicable diseases (CNCDs) are progressively assuming greater importance. Of the 35 million deaths from chronic disease in 2005, 80% occurred in low- and middle-income countries, where the majority of the world’s population lives.[9]
Changes in risk factors and lifestyle also contribute. Latin America exemplifies stage three in the health transition: life expectancy is increasing, and high-fat diets, smoking, and sedentary lifestyles are becoming more common. Consequently, cardiovascular disease, including CVD, is becoming one of the most prominent public health problems, to a lesser degree than in stage two regions (China and India), where lifestyles and risk factors have yet to make a similar impact, or in stage four regions (such as Europe), where health strategies and lifestyle changes have reduced CNCD risk factors.[3,4,9]
At present, Cuba has the second oldest population in Latin America; 16.6% of the population is aged ≥60 years.[6] This means that diseases related to aging, such as stroke, will increase, posing a challenge to the health system. Determining stroke prevalence is important for defining health strategies and making evidence-based decisions for resource management.
Few population studies have been carried out on the prevalence and incidence of cerebrovascular diseases in Cuba. Data on stroke prevalence is found in the registries of the national health statistics system and in results of a 2002 self-report disease survey.[10]
However, Cuba is part of the 10/66 Study, designed to estimate the prevalence and incidence of dementia and other CNCDs, including stroke, in the population aged ≥65 years.[11] The study protocol has involved three phases: a 2001 pilot study to validate the 10/66 instruments and diagnostic algorithm in 25 countries, including Cuba;[12] a single-phase, door-to-door cross-sectional “prevalence study” of adults aged ≥65 years, carried out in Cuba in 2003–2006 with a total sample of 3015 individuals located in catchment areas of selected community-based polyclinics in Havana City and Matanzas provinces; and a 3-year follow-up of the cross-sectional study populations in seven Latin American countries (including Cuba) and China with 15,000 participants, which will conclude in 2010. Details of this research protocol have been widely published.[11,13,14]
The present paper is the first report on stroke prevalence and its associated risk factors in adults aged ≥65 years from selected urban areas of Havana City and Matanzas provinces, as part of the 10/66 population-based study.
METHODS
A single-phase, descriptive, door-to-door cross-sectional study of adults aged ≥65 years, also known as a prevalence study, was carried out from June 2003 to December 2006.
Techniques and procedures The universe consisted of all adults aged ≥65 years residing in selected municipalities of Havana City and Matanzas provinces in January 2003. A sample of 3015 older adults was chosen using multi-stage cluster sampling.
First-stage sampling units were five municipalities in Havana City Province (Marianao, La Lisa, Playa, Plaza, and 10 de Octubre) and the city of Matanzas in the province of the same name. Second-stage units were catchment areas of selected polyclinics in each municipality: 27 de Noviembre, Ramón González Coro, and Carlos Manuel Portuondo polyclinics in Marianao; Cristóbal Labra polyclinic in La Lisa; Ana Betancourt polyclinic in Playa; 19 de Abril polyclinic in Plaza; 14 de Junio polyclinic in 10 de Octubre; Milanés Polyclinic in Matanzas City. Third-stage sampling units were catchment areas of neighborhood family doctor-nurse offices located <500 meters from the selected polyclinics. The study universe was the entire population aged ≥65 years residing <500 meters from the selected polyclinics.
Study implementation was coordinated at the municipal level and with the selected polyclinics and family doctor offices, which facilitated collecting the information necessary to fulfill the study objectives.
Every household in each doctor-nurse office catchment area was visited to conduct a census of the population and produce a map of the territory covered, listing all persons aged ≥65 years. The following data were collected and used to create the participant registry database: first and last names, age, sex, participant identification number, address, and contact information for two neighbors or other key contacts.
Based on an assumed prevalence of 6.5% used in previous studies of cerebrovascular disease, a sample size of 3015 adults aged ≥65 years was calculated, with a precision of ±0.8%. The sample comprised 2100 older residents of Havana City Province and 915 older residents of Matanzas Province.
Ethical procedures: Verbal and written consent was requested from the older adults selected, or alternatively, approval of their caregivers. All data collected in interviews was kept confidential. The study protocol was approved by the Ethics Committee of the Medical University of Havana.
Interviewer preparation: A procedures manual was written covering all aspects of the study, including training and field procedures. The interviewers were 12 physicians, either clinical specialists or psychiatrists, who participated in the baseline study using the 10/66 protocol.[11,13] All interviewers received one week of extensive training on the instruments and surveys to be applied.
Instruments and variables: Interviews were conducted in participants’ homes. The interview and application of the instruments took an average of 2–3 hours.
The 10/66 protocol questionnaire included a structured interview with participants covering sociodemographic characteristics, health status, lifestyle, and risk factors; a physical and neurological exam; and an interview with a reliable informant.[11,13] All materials, questionnaires, and assessments were provided by the 10/66 Study. Before application, they were translated from English into Spanish by two bilingual translators (one clinician knowledgeable on the topic and another who was not), the final version was discussed and approved by a coordinating committee after it was back translated and administered to a sample of 120 subjects. A research procedures manual and training video were prepared on the physical and neurological exam.
For the purposes of this study, the following variables were included:
- Sociodemographic characteristics: age, sex, educational level.
- Self-reported CNCDs based on a standardized questionnaire that included questions such as, “Have you ever been told by a doctor that you had a stroke / heart attack / angina / diabetes?” and a description of episodes.
- Smoking: smoker, ex-smoker, non-smoker, and length of exposure; type of tobacco used (cigarettes, cigars, pipe tobacco, chewing tobacco, snuff); average number of units used per day; age at start of habit and age when the person quit, if habit was interrupted.
- Alcohol consumption: number of units ingested per week, before and after age 65. To determine alcohol dependence, a cutoff level was established: 14 units/week for women and 21 units/week for men. The maximum usual consumption per week was recorded in units of alcohol, by type of drink: one glass of beer (250 ml = 2 units), one shot of liquor (22 ml = 2 units), or one glass of wine or sherry (175 ml = 2 units), and one bottle of liquor (1000 ml = 32 units). Participants were asked, “Has there ever been a period of several years when you would have said that you were a heavy drinker? Have you ever had treatment or help for drinking from a doctor or some other agency?”; and the interviewer was asked to record his or her opinion about whether alcohol was a problem for the participant before age 65.
- Mean systolic and diastolic blood pressure, measured on two occasions, sitting and standing. Two methods were used for diagnosis of hypertension: participant’s self-report (“Have you ever been told that you had high blood pressure? When were you first told? Were you started on treatment? Are you still on treatment?”) and/or blood pressure readings that met European Society of Hypertension (ESH) cutoff points (systolic pressure ≥140 mm Hg and/or diastolic pressure ≥90 mm Hg).[15]
- Laboratory tests (normal reference values in parentheses). Complete blood count: hemoglobin (men 13.0–17.0 g/100 ml; women 12.0–16.0 g/100 ml); hematocrit (men 41.0–53.0; women 36.0–46.0); mean corpuscular hemoglobin, MCH (26.0–34.0 pg/cell); and fasting glucose (4.2–6.4 mmol/l). Lipid profile: total cholesterol (3.5–6.2 mmol/l); lipoprotein fractions: high-density lipoprotein, HDL (>0.9 mmol/l); very low density lipoprotein, VLDL (<1.04 mmol/l); triglycerides (<1.86 mmol/l). Commercial reagents were used (Roche, USA). Low-density lipoprotein (LDL) concentrations (<3.4 mmol/l) were calculated using the Friedewald formula.
- Presence of the apolipoprotein E (APOE) genotype and its three isoforms: APOE ε2, APOE ε3, and APOE ε4. Polymerase chain reaction (PCR) was used, following standard protocol.
- Stroke diagnosis according to WHO definition. Information was obtained from the participant and a reliable informant about: a) sudden or rapidly developing clinical signs of focal (or global) neurological dysfunction, lasting more than 24 hours, with no apparent non-vascular cause (brain trauma, neoplasia, coma attributable to a metabolic disorder or hydroelectrolyte imbalance, vasculitis, central nervous system infection, or peripheral neuropathy).[16]
All individuals surveyed were given a structured physical and neurological exam allowing objective, quantitative measurement of focal signs, parkinsonism, ataxia, apraxia, and primitive reflexes (NEUROEX),[17] using the US National Institutes of Health Stroke Scale (NIHSS) to pinpoint stroke signs and symptoms.[18] If a participant had had an imaging study (Computerized Axial Tomography–CT Scan or Magnetic Resonance Imaging–MRI), availability of written information about the study was noted, as well as time elapsed following the event before the study, and the neuroimaging findings.
A report was prepared summarizing findings of the clinical history, tests performed, and records obtained from two independent physicians in addition to the interviewer, responding to the following questions:
- Did the participant have a stroke?
- If yes, when?
- Can the event be localized?
- Can it be determined whether it was hemorrhagic or ischemic?
Data processing and analysis Stroke prevalence was estimated by sex and age group. Crude and age- and sex-adjusted prevalence ratios were calculated, with 95% confidence intervals, using a Poisson regression model.[19]
Because this was a cross-sectional study, analysis focused on risk factors and their association with stroke, considering only those risk factors or exposures that occurred at least 3 years prior to the stroke, to decrease the possibility of inverse causality.
To determine the influence of a group of risk factors on the presence of stroke, a univariate analysis was initially performed between each of the explanatory or independent variables (qualitative) and the response or dependent variable (stroke). A multivariate analysis then included the variables that demonstrated a significant association (p <0.05), using the test of independence (χ2) or according to expert opinion.
To prevent excessive correlation among independent variables (colinearity) from skewing estimates, association was evaluated by the test of independence (χ2) for qualitative variables, accompanied by a correlation coefficient (Phi or Cramer’s V, depending on whether contingency tables used 1 or more degrees of freedom), and by Pearson’s linear correlation coefficient for quantitative variables. The correlation was considered important if the coefficient value was >0.8. Subsequently, multiple logistic regression for dichotomous responses was used.
Analyses were performed using Stata version 9.2 (StataCorp 2007, Stata Statistical Software: release 10; StataCorp, College Station, TX). The numbers of missing values are described for each variable used in the analyses. For multivariate analyses, only participants with complete data for all independent variables were included.
RESULTS
Of the 3015 individuals selected, 2944 interviews were completed (97.6% response rate); 2043 in Havana City Province (97.2%) and 901 in Matanzas Province (98.4%).
Mean age was 74 years; 25.6% of the sample was aged ≥80 years, and 65.0% was female. Educational levels were relatively high: 75.2% of those surveyed had 7 or more years of schooling and 16.9% had a university degree. There was a high prevalence of cardiovascular risk factors and CNCDs; 51.0% of participants reported a history of hypertension and 55.6% met ESH hypertension criteria; 18.6% had prior diagnosis of diabetes mellitus; and 30.7% reported history of ischemic heart disease diagnosed by a specialist (Table 1).
The samples in Havana City and Matanzas provinces were homogeneous with regard to sociodemographic characteristics, health profiles, and lifestyle (data not presented).
Stroke prevalence Overall stroke prevalence was 7.8% (95% CI 6.9%–8.8%). The precision of the intervals demonstrates the quality of the estimates obtained. Stroke prevalence increased with age, with the exception of the group aged 75–79 years, which was slightly lower than the group aged 70–74 years. Prevalence was greater in men (9.5%) than in women (7.0%); the overall male/female prevalence ratio was 1.36 and was much higher in the group aged ≥80 years (1.61) (Table 2). In 56% of cases, diagnosis was confirmed by CT Scan or MRI.
Table 1: Risk Factors Associated with Stroke, 10/66 Study in Cuba (n = 2944)

a Adjusted for all the co-variables in the model b Meets European Society of Hypertension criteria c Self-reported d Anemia (Women Hb <12 g/100ml / Men Hb <13 g/100 ml) CI: Confidence Interval ref.: reference
Stroke risk factors In the univariate analysis (Table 1), stroke was associated with increased age, male sex, smoking, history of alcoholism, hypertension, diabetes mellitus, ischemic heart disease, and being a carrier of 1 or 2 APOE ε4 alleles. The adjusted prevalence ratio showed a similar pattern.
In the population studied, the probability of suffering a stroke was significantly influenced by a history of high blood pressure, low HDL cholesterol levels, male sex, anemia, self-reported heart disease, carrier of 1 or 2 APOE ε4 alleles, and age (Table 3).
History of high blood pressure had the highest odds ratio (OR 2.8) among the dichotomous qualitative variables, which means that hypertensive older individuals are almost three times more likely to suffer a stroke than their normotensive counterparts. Low HDL cholesterol had an OR of 2.6. Male sex had an OR of 1.7, and, in decreasing order, history of anemia, heart disease, and homozygosity or heterozygosity for the APOE ε4 genotype showed significant associations.
With age (quantitative variable), an OR of 1.3 was found, which means that the probability of stroke in an older person is 1.3 times greater with each passing year.
Of 157 strokes diagnosed in the interviews in Havana City Province, 145 were classified ischemic (92.4%) and 12 hemorrhagic (7.6%). In Matanzas Province, of 72 strokes, 68 were classified ischemic (94.4%) and 4 hemorrhagic (5.6%).
DISCUSSION
This paper presents some of the first research results on stroke prevalence in the Cuban population from the 10/66 population-based study. The strategy of surveying the entire population within the selected area raised the response rate and has facilitated subsequent longitudinal follow-up study.
Previous reports of stroke prevalence in Cuba, based on a continuous recording method that includes medical statistics and departmental clinical records in all health care institutions in the country, show a steady increase between 1995 and 2006.[10]
In the Second National Survey on Risk Factors and Chronic Diseases, conducted in 2002, the case definition of stroke was based on an individual’s self-report of having been diagnosed at some time. Overall prevalence found in that study was 260 per 100,000 population and 850 per 100,000 population for the group aged ≥60 years.[20]
With regard to the strategy for detecting a positive case of stroke, recording a history of stroke based on a single question results in a high rate of false negatives, while the use of a stroke symptom questionnaire has a high rate of false positives.[21] A study in Germany estimated stroke prevalence at 4.5% based on a history of stroke according to medical diagnosis; when individuals who reported major stroke symptoms were added, the estimate rose to 7.6%.[22]
The Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study, a national population-based longitudinal study in the United States, found that 7% of patients had a clinical diagnosis of cerebral infarction and transient ischemic attack (TIA), and an additional 18% of patients reported stroke symptoms but had not received a medical diagnosis.[23]
Table 2: Prevalence of Stroke by Age Group and Sex, 10/66 Study in Cuba
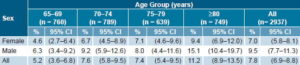
CI: Confidence Interval
Table 3: Association between Stroke and Selected Risk Factors
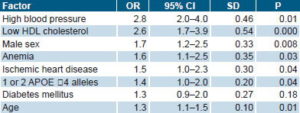
OR: Odds Ratio CI: Confidence Interval SD: Standard Deviation
In our case, the diagnosis of stroke is based on the self-report of the individual and a reliable relative, a structured survey to identify focal signs and symptoms of neurological dysfunction, and a structured physical and neurological exam by a physician, with final diagnosis established by two independent clinicians, resulting in a more precise clinical diagnosis.
In the present study, stroke prevalence in the population aged ≥65 years was similar to that reported for the same age group in an urban area of Madrid (8.5%) and in a rural area of Ávila, Spain (7%), but was almost twice that reported in a door-to-door study in a rural area of Girona, Spain (4%).[24] Another study in Spain found a prevalence in older adults of 6.4%: 7.3% in men and 5.6% in women; 8.7% in urban areas vs. 3.8% in rural areas,[25] and a summary of five studies in adults aged ≥65 years in Spain found an estimated prevalence of 7.5%.[26] In four regions of the United States estimated prevalence in the population aged ≥65 years was 4.7%, while in L’Aquila, Italy, it was 7.3%.[27]
In Latin America, stroke prevalence in older adults varies from 1.9% to 4.8%.[7] Most population-based studies were done in the 1990s using small sample sizes and definitions of stroke, age groups, and methodology too different to enable comparisons. Estimated prevalence in Vassouras, Brazil, was 2.9%, but the method for detecting positive cases was based on medical records and verification by clinical examination.[7] Since 2000, we found only one study, in Atahualpa, Ecuador, in 2004, which found a crude prevalence of 638 per 100,000 population aged ≥15 years.[8] Most studies refer to individuals aged ≥60 years.
The pattern of stroke in Cuba is similar to that reported in various European countries and in North America.[7,22,23,27] In Latin America and the Caribbean, several infectious diseases are often seen in association with stroke.[27] This is the case with Chagas disease (American trypanosomiasis), which causes cardiomyopathy, and neurocysticercosis,[28,29] which can produce small- and large-vessel cerebral arteritis. These diseases are not found in Cuba.
It should be noted that in the present study, current WHO diagnostic criteria for stroke were used, which include clinical manifestations lasting more than 24 hours.[16] This constitutes a limitation of this and almost all prevalence studies, since the concept of TIA was modified in 2004 to use a shorter therapeutic window (1 hour) or consider the absence of lesions in neuroimages.[30] If we include patients with symptoms of less than 24 hours duration, the prevalence would certainly be higher.
Lack of diagnostic confirmation of all cases by CT Scan or MRI is also a limitation of this study, since stroke diagnosis was based on clinical data in a large percentage of cases. Therefore, subjects who had had silent cerebral infarctions without symptoms or focal signs were probably excluded. Positive case definition using neuroimaging, particularly MRI, would eliminate biases related to transient ischemic symptoms or stroke-like episodes. This cannot be done in practice, however, even in highly developed countries, due to cost and lack of accessibility.
A review of epidemiological studies found a male/female prevalence ratio for stroke of 1.41, meaning that stroke is 1.41 times more frequent in men than in women.[31] In the present study, this ratio was 1.36, indicating that prevalence in men was likewise greater.
One striking aspect is the very small proportion of patients with hemorrhagic stroke: 7.6% in Havana City Province and 5.6% in Matanzas Province. In studies based on hospital records in developed countries, the proportion of hemorrhagic stroke is 10%.[32] In the Oxford Vascular Study, from 4.5% to 5.0% of small strokes, with fewer than three points on the NIHSS, were intracranial hemorrhages.[33]
The risk associations reported in this study (age, sex, and cardiovascular risk factors) are similar to those reported in other studies. Heart disease is the third most important risk factor for stroke, after age and high blood pressure. Atrial fibrillation is a prime cardiac risk factor, since it quadruples the risk of stroke in the general population, followed by heart failure, which doubles or triples the risk.[34]
Another limitation of this study is that electrocardiograms were not done on all participants; even though cardiovascular auscultation was performed, the information obtained may be skewed by observer bias. This might explain why the association between ischemic heart disease and stroke was smaller than that reported by the Framingham Heart Study, in which ischemic heart disease tripled stroke risk. Ischemic heart disease is frequently associated with other risk factors, such as hypertension, diabetes, and/or atrial fibrillation.[35]
Our study found an association between having 1 or 2 apolipoprotein E4 alleles and the risk of stroke. It is reported that subjects with the APOE ε4 genotype have more severe coronary atheromatosis;[36] in addition, the genotype has been associated with an increased risk of cerebral infarction from large-vessel atheromatosis and lobar intracerebral hemorrhage.[37] A meta-analysis of 22 studies found an association between the APOE ε4 genotype and greater carotid intima-media thickness,[38] a predictor of large-vessel atheromatosis.
Post-stroke dementia is common. Close to 25% of stroke survivors develop dementia.[39] Possible mechanisms for this include characteristics of the vascular lesion (extent and location), comorbidity with Alzheimer’s disease, changes in white matter, cerebral hypoperfusion, functional disconnection, interaction with APOE ε4, or a combination of these mechanisms.[40] Since the early 1990s, APOE ε4 has been widely recognized and confirmed as a genetic risk factor for late-onset Alzheimer’s disease;[40] however, its association with stroke has been less studied.
The relationship between anemia and stroke is well established for sickle-cell anemia.[41] However, some studies have associated iron-deficiency anemia with stroke risk, especially in childhood.[41] The few studies that have looked at the relationship between low hematocrit and risk of ischemic stroke have had conflicting results.[42–45] However, it has been demonstrated that anemia is one of the predictors of recurrence in stroke patients with internal carotid artery occlusion.[46] Furthermore, anemia contributes to the worsening of ischemic white matter lesions in hypertensives[47] and is a factor associated with cognitive decline in old age,[48] which could provide an explanation for the association between anemia and stroke reported in our study.
Given that this was a cross-sectional study, definitive research on the causal association of these factors with stroke occurrence awaits the outcomes of the longitudinal study to be completed in 2010.
CONCLUSIONS
Stroke prevalence in adults aged ≥65 years is similar to that reported for European and North American countries and higher than that observed in Latin American countries. The risk profile includes classic risk factors plus anemia and APOE ε4 genotype. Further studies to examine these differences more closely, including the role of vascular risk factors and the association between APOE ε4 genotype and stroke, are recommended.
ACKNOWLEDGMENTS
This study is part of the 10/66 Dementia Research Group population-based research program in Cuba, a collaborative agreement between the London Institute of Psychiatry and the Medical University of Havana, sponsored by the Wellcome Trust Foundation (GR066133) and the Cuban Ministry of Public Health. We thank all the researchers who took part in this population-based study.






