INTRODUCTION
Cancer is a major public health problem worldwide,[1] with 17.5 million cases and 8.7 million deaths in 2015.[1,2] Approximately one third of cancer patients develop a brain metastasis (BM).[3,4] BM is 10 times more frequent than primary brain cancers.[5] It is the most frequent tumor of the central nervous system (CNS) and has a very poor prognosis in most cases.[5,6]
BM incidence has increased worldwide 2–5 times in the past 40 years, despite scientific and medical advances.[5] This increase can be explained by the following: increased cancer patient survival, which gives more time for metastases to appear;[7,8] improvements in diagnostic imaging with the advent of computed tomography (CT) and magnetic resonance imaging (MRI), allowing identification of increasingly smaller metastases;[9] increased lung cancer and melanoma incidence;[6,10] general population aging; the fact that most systemic chemotherapeutic agents do not cross the blood–brain barrier; and finally, that some chemotherapeutics weaken this barrier, which facilitates entry of malignant cells into the CNS.[10]
According to official health statistics, cancer was the second cause of overall mortality in Cuba in 2016, with an incidence rate of 216.3 per 100,000 population,[11,12] thus a high burden of BM can be expected. Most Cuban research on the subject consists of autopsy studies and hospital case series, rather than population studies.[13–18] The most extensive study was carried out in 2014,[16] but only included patients admitted to selected hospitals, which did not permit determination of population prevalence or relative frequency of BM among cancers. After an exhaustive search of national publications in PubMed and SciELO databases, as well as Cuba’s National Cancer Registry,[12] using the search terms “brain metastasis/Cuba/epidemiology/incidence/prevalence,” five articles were identified, two autopsy studies[14,15] and three hospital case series.[16–18] No population studies of BM were found.
Population studies only appear in the international literature, along with some mistakenly classified as such. For example, in 2002 an epidemiological study of BM based on hospital populations in Aragón and La Rioja, Spain, was published.[19] On the other hand, true population studies are scarce, among them Barnholtz-Sloan’s 1973–2001 cohort study of 16,210 US cancer patients,[20] and Schouten’s 1986–2005 cohort study in the Netherlands.[21] These two studies constitute our main external referents.
Due to the paucity of epidemiological studies on the subject, our study aimed to characterize patients with brain metastases residing in Habana del Este Municipality, in Havana, Cuba, with respect to demographic indicators, metastasis location and primary tumor.
METHODS
Study type and population A retrospective descriptive study was carried out based on data for patients residing in the municipality of Habana del Este in Havana, Cuba who were diagnosed with cancer in 2014. This municipality was selected to allow comparison with a previous study in the Luis Díaz Soto Hospital (serving a large part of the Municipality’s population), which gathered the largest series of autopsies in Cuba.[15] In Cuba’s National Health System (NHS), primary health care (PHC) is delivered in family-doctor-and-nurse offices (CMF), and multispecialty community polyclinics to which CMFs report.[22] Habana del Este has 24 polyclinics and 192 CMFs. In Cuba, cancer patients receive special diets through PHC and are continuously linked with CMFs in office and home visits, even while being treated in secondary or tertiary care.
Inclusion and exclusion criteria Patients with histologically confirmed cancer diagnoses, treated at any level in the NHS were included. Patients with primary hematopoietic neoplasms (leukemias, lymphomas) were excluded, since in such cases, infiltration of the leptomeninges causes BMs with different biological and pathological characteristics.
Terminology
- Patients with BM: those with imaging confirmation (CT or MRI)
- Supratentorial: located above the cerebellar tentorium
- Infratentorial: located below the tentorium
- Cortico–subcortical: located in the cerebral cortex or immediately adjacent
- Synchronous metastases: diagnosed at same time as primary tumor
- Metachronous metastases: occurring months or years after primary tumor diagnosis
Variables
- Age in years at time of BM diagnosis, grouped as categorical variable: 20–40, 41–60, >60
- Sex: male, female
- Skin color: white, black, mestizo
- Primary site: organ where primary tumor was located
- BM location: frontal, parietal, temporal, occipital lobes; cerebellum; brainstem
- Number of metastases: 1, 2–5, 6–10, >10
- Control of primary tumor: controlled, uncontrolled (with residual lesions)
- Extracranial metastases: present, absent
Data collection Initially, we visited the municipal Office of Food Supply Control (OFICODA), which distributes cancer patients’ special diets, to obtain the number of patients receiving such diets, and the Municipal Health Department of Habana del Este, which maintains health statistics about its population. The number of patients with cancer was obtained from these sources, as well as data on demographics (age and sex), on diet requisitions, and the numbers of patient’s CMFs and community polyclinics.
Thus, we were able to contact the corresponding CMFs by phone to obtain the remaining information needed. When this was not possible, we visited patient residences personally or contacted them by telephone. Patients with a history of BM were visited at home and clinical histories were reviewed at the clinical–surgical hospitals or the research and care institutions where they were diagnosed and treated.
From these records, information was obtained on imaging studies (CT and/or simple or contrast cranial MRI). A data collection form was used (Appendix) and data were then transferred to a Microsoft Excel 2010 table.
Analysis Cancer and BM prevalence was calculated as the number of patients diagnosed with each in 2014, over the population of Habana del Este Municipality obtained from the 2014 population and housing census (181,473), multiplied by 100,000. Relative frequency of BM for each site was calculated as the proportion of patients with BM among the total number of cancer patients for that site the same year. Data were organized in frequency distribution tables. Absolute and relative frequencies were used.
Ethics Patients with BM received all the necessary information about the study before been asked to provide written consent to participate. The study protocol was approved by the Habana del Este Municipal Health Department’s Ethics Committee and authorization was obtained to access data from OFICODA. Data management procedures protected patient confidentiality.
RESULTS
There were 832 persons diagnosed with cancer in 2014, for a prevalence of 458.5 per 100,000 population; of these, 27.6% (230/832) had malignant brain neoplasms, 83% (191/230) of which were BMs and 17% (39/230) primary, a ratio of 4.9:1. Relative frequency of BMs among all cancer patients was 23% (191/832), for a prevalence of 105.2 per 100,000 population. Melanoma had the highest relative frequency of BM, 77.4% (Table 1).
Table 1: Relative frequency of brain metastases by primary site, Habana del Este, 2014
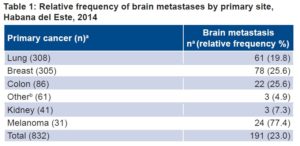
apatients
bIncludes two patients with brain metastases from adenocarcinoma of the prostate and another from laryngeal adenocarcinoma.
The largest age group among BM patients was aged 41–60 years (48.2%, 92/191); there were no patients aged <20 years with BM. Some 61.3% (117/191) of BM patients were female. Relative frequency was similar among white, black and mestizo patients (36.6%, 29.8% and 33.5%, respectively). Breast and lung were the primary sites in 72.8% of BM patients, breast being the most frequent site in women (66.7%, 78/117), and lung in men (50%, 37/74) (Table 2).
Table 2: Demographic characteristics of patients with brain metastases by primary site, Habana del Este, 2014
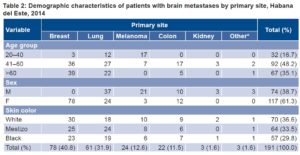
*Includes two patients with brain metastases from adenocarcinoma of the prostate and another from laryngeal adenocarcinoma
BMs from melanoma were more frequent in men than in women (28.4% vs. 2.6%). Of patients with other primary sites, two BMs originated in prostate adenocarcinoma and one in laryngeal adenocarcinoma. No association with primary site was found for age group and skin color (Table 2).
Almost half (46.8%) of all BMs were in the parietal lobe. All BMs secondary to kidney cancer were in the cerebellum, but there was no association between location and primary site (Table 3).
Table 3: Location of intracranial brain metastases by primary site, Habana del Este, 2014 (n = 451)a
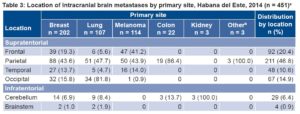
aSome patients had >1 metastasis.
bIncludes two patients with brain metastases from adenocarcinoma of the prostate and another from laryngeal adenocarcinoma.
Table 4: Number of patients with brain metastases by primary site, 2014 (n = 191)
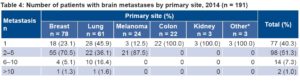
*Includes two patients with brain metastasis from adenocarcinoma of the prostate and another from laryngeal adenocarcinoma.
In 59.6% of BM patients there were multiple lesions, the majority (91.6%) having <6. All BMs of colon, kidney and “other” sites were solitary (Table 4).
Residual lesions in the primary site were observed in 98 patients (51.3%), despite cancer treatment, while 87 (45.5%) had extracranial metastases.
DISCUSSION
True BM prevalence is difficult to determine in clinical and hospital series, since many metastases are not diagnosed during life and autopsy studies have selection biases; hospitalized patients are not necessarily representative of the population. Not all patients diagnosed with BM are admitted to a hospital or brought to autopsy.
That motivated our population-wide study, which is more reliable, has fewer biases and is more representative of the population. The data source, OFICODA, is highly reliable, because virtually all cancer patients receive the special diets it distributes. Data capture methods employed cannot guarantee 100% coverage or bias-free information, but data are more complete than would be obtained in hospital or autopsy-based studies. Furthermore, the retrospective design allowed us to obtain and process selected variable data in a relatively brief time.
The relative frequency of BM in cancer patients (23%) is in the range found by Grossman (10%–40%),[3] but higher than in epidemiological studies by Barnholtz-Sloan and Schouten, in which prevalence for all primary sites combined was 9.6%[20] and 8.5%,[21] respectively. However, these two studies included only certain cancers (lung, breast, melanoma, colorectal and kidney). In most cancer patients, routine imaging studies are not performed, and many metastases may remain asymptomatic; therefore, theoretically the true prevalence of BM is greater than that found in epidemiological studies.
Of the two epidemiological studies we retrieved, Schouten did not examine skin color,[21] while Barnholtz-Sloan observed significantly higher incidence proportions in African Americans compared with white patients for lung, melanoma and breast cancer; similar for colon cancer; and lower for renal cancer.[20] We were unable to stratify all cancer patients by skin color and calculate comparative BM risk from relative frequency, which excludes any reliable inference about an association between skin color and BM risk. However, skin color distribution in BM cases is not suggestive of an association.
BM constitutes a high proportion of CNS neoplasms in autopsy studies, surpassing primary brain neoplasms by 10:1,[5,6] double what we observed. This could be explained by differences, discussed earlier, between population studies and hospital case series and autopsy studies. In Cuba (as elsewhere), underdiagnosis or underregistration of metastases may reduce numbers seen in population studies, while autopsy studies may be able to detect even micrometastases.
BM patients are usually older, with peak incidence between ages 50 and 60 years,[5] in keeping with the higher frequency of cancer in these age groups. Thus, the predominance of age >40 years in our study is not surprising.
BM incidence tends to be similar for men and women, with slight predominance in men, (except for breast cancer, which is very rare in men).[6] The predominance of women in the BM group we studied reflects the high numbers of breast cancers and small numbers of cancers that are more frequent in men, such as prostate and colon cancers.
Some neoplasms tend to develop brain metastases more than others. The “seed and soil” hypothesis describes one possible biological mechanism, that some neoplasms tend to develop metastases in certain target organs through molecular mediators and membrane receptors.[23–26] Testicular cancer, melanoma, lung cancer and renal cell carcinoma display the greatest propensity for BM, in order of frequency.[15] On the other hand, other lesions such as prostate and stomach cancer rarely metastasize to the brain.[14,15] The high relative frequency of BM we observed for melanoma is consistent with reports from other authors.[20,21]
The lung is the most common primary site for BM in most hospital and pathology series[14,15,17] and in epidemiological studies.[20,21] This is reflective of its higher incidence as primary tumor as well as its propensity to metastasize. In two autopsy studies of Cuban adults, the most frequent origins of BM were lung cancer (50%–60%), breast cancer (15%–20%), skin cancer (5%–10%) and cancers of the GI tract (4%–6%).[14,15]
However, in our study, breast was the predominant site for BM, followed closely by lung cancer. This phenomenon could be explained by the high proportion of breast cancer patients with BM found in the study population, perhaps related to the current low survival of patients with lung cancer compared with those of breast cancer, who experience longer survival thanks to advances in early diagnosis and current therapies that increase time available for metastasis to occur.[26,27] Interestingly, there were only 3 more cases of lung cancer than there were of breast cancer in our series; in 2013, there were 5722 new cases of lung cancer and <4000 new cases of breast cancer in Cuba.[11] This difference could also reflect lower lung cancer survival, since our series examined prevalent cases.
Our findings regarding BM location are consistent with observations elsewhere that most BMs are supratentorial. Between 60% and 80% of intracranial metastases are supratentorial, with 20%–40% infratentorial (15% cerebellum and 5% brainstem).[6] Cortico–subcortical location in the frontal, parietal and temporal lobes has been explained by vascular and molecular factors, since it is the distribution area of the middle cerebral artery, which has the largest caliber of terminal branches of the internal carotid artery. Thus, tumor emboli are more likely to be directed to this artery.[6,25]
Adenocarcinoma of the breast and colon, renal cell carcinoma and thyroid carcinoma are known to produce single BMs, while melanoma and lung cancer tend to produce multiple BMs.[6] In Cuban autopsy studies, more than half of cancer patients have single metastases.[14,15] While single BMs were frequent in our study, most frequent were patients with 2–5 lesions. International research reports frequent multiple BMs in breast cancer, partly because longer survival provides more time for patients to accumulate risk of new metastases.[26] Also, the brain is a propitious location for breast cancer cells, since BMs are not affected by chemotherapeutic agents and monoclonal antibodies, principally because of the blood–brain barrier. Patients with HER2 positive and triple-negative breast cancer (negative for estrogen, progesterone and HER2 receptors) have increased BM risk.[28,29]
Treatment with trastuzumab has been shown to act on extracranial metastases, but not on intracranial ones, thereby “unmasking” the latter.[30,31] Our retrospective design and limited available data prevented us from obtaining insights on this point.
In our series, multiple breast metastases predominated, more than half of breast cancer patients having oligometastasis. Oligometastasis may have a better prognosis than a larger number of lesions, if the primary lesion is controlled and metastases treated focally.[8,30,32] Some authors arbitrarily use the term “extensive metastases” to refer to presence of ≥10 metastases.[25,27,28] In our series, only two patients, having primary breast and lung cancer, respectively, were found with ≥10 lesions.
Just over half of patients had uncontrolled primary cancer and almost half had extracranial metastasis, something that has not been reported in previous Cuban studies.[14,15] This substantially worsens their prognosis, because an uncontrolled primary lesion limits options for specific BM treatment.[29] In Nieder’s study, 32% of patients had uncontrolled primary disease (consistent with our study) and 77% had extracranial metastasis,[6] a higher percentage than we found.
Our results are useful as a starting point to approach BM as a health problem. However, they should be interpreted cautiously, because of some study limitations. In the first place, this was a descriptive retrospective study, without control of relevant variables that can be assessed in prospective studies, such as overall survival, local control and disease-free survival. In addition, because the study was based on administrative data, it could not have the rigorous data standardization of a clinical research study. For example, when contrast media were not available, imaging results could have been subject to bias, if some metastases were missed.
Furthermore, data on disease states at the municipal level might not be representative of the national situation. Presence of asymptomatic metastases can lead to underestimates of true prevalence in population studies, while at the same time, there can be false positives because of concomitant nonmetastatic lesions. Nonetheless, the advantage goes to population-based studies over autopsy series or hospital studies, for the reasons enumerated earlier.
A highly developed PHC system in Cuba, based on CMFs reporting to community polyclinics, ensures that medical attention and services are accessible to the entire population. There have been advances in complementary detection methods with increasingly higher sensitivity and specificity and increasingly targeted therapies (such as radiosurgery). Deployment of BM imaging studies in cancer patients on an epidemiological scale has an unacceptably high cost–benefit ratio.[5] Together these factors tend to reduce the role of PHC in early detection of metastases.
Nevertheless, early neurological BM signs might be detectable in PHC if physicians maintain a sufficiently high index of suspicion. Such timely detection could lead to earlier referral to other care levels for confirmation and interventions to improve quality of life and survival.
CONCLUSIONS
Brain metastases are more prevalent in this Cuban municipality than reported in other countries, but they constitute a higher proportion of cancer cases than seen in other population-based studies. The study’s results underline the importance of detecting brain metastasis early, to permit timely interventions to improve quality of life and survival.
APPENDIX 1








