INTRODUCTION
Fetal macrosomia (birth weight >4000 g at term) is the most important complication in newborns of mothers with diabetes mellitus, whether preconceptional or gestational (diagnosed during pregnancy, especially after 24 weeks). This complication is usually associated with other problems in gestation and labor, the most important being birth trauma, sepsis, and respiratory, cardiovascular, metabolic or hematologic disorders.[1,2]
Worldwide, the frequency of fetal macrosomia in infants of mothers with gestational diabetes (IMGD) is approximately 10%–30%, and, among other things, reflects the quality of obstetrical and endocrinologic care received by women with gestational diabetes (GD) during pregnancy.[3,4] In Cuba, this complication occurs in approximately 12%–20% of IMGDs.[5,6]
Quality prenatal care during pregnancy (including quality primary care) makes prevention of macrosomia in IMGDs possible by ensuring early detection of related conditions. There is evidence that fetal macrosomia in IMGDs is generally associated with certain maternal conditions, such as previous macrosomic child, older age, initial overweight or obesity, excess weight gain during pregnancy, late diagnosis of GD, poor glycemic control, hypertriglyceridemia and prolonged pregnancy.[4,6,7]
The diagnosis of fetal macrosomia in IMGDs should be done as early as possible, that is, at about 26 weeks’ gestation, to begin early therapy and thereby minimize risk of poor maternal and perinatal outcomes. Early diagnosis of fetal macrosomia is also the only course available when no previous mitigation of avoidable risk factors has been accomplished.[4,6,7]
Excessive growth in macrosomic IMGDs depends mainly on uneven increase in fetal abdominal circumference (FAC) and thoracic and biparietal diameter, resulting in a high thorax/head ratio. This is mainly due to excess subcutaneous fat accounting for 20% of the infant’s body weight (compared to 12% in newborns of normal weight). In fact, during the last weeks of pregnancy, the fetus of a mother with GD usually deposits 50%–60% more fat than the fetus of a nondiabetic mother. On the other hand, the increased FAC also reflects enlarged abdominal organs, especially hepatomegaly (typical of the IMGD with macrosomia).[3,4] This has led some researchers to propose that IMGD fetal weight should be estimated from FAC determined by ultrasound (US), rather than from the weight estimate generated by US software. They believe this approach would provide a more sensitive measurement, which, depending on its value, could more accurately predict macrosomia.[3,8]
Fetal US has estimated 70% sensitivity and specificity for predicting macrosomia in newborns.[3] The mean error is 200 g for use of US for FAC measurement and fetal weight estimation for gestational age in specific curves or tables relating both fetal parameters. Periodic measurement of this US parameter must start at 26–28 weeks’ gestation and be carried out at intervals of 21–30 days. US-determined FAC at the >75th percentile at the beginning of the third trimester has been associated with fetal macrosomia in IMGDs.[3,8]
The adverse effects of macrosomia on IMGDs are not only restricted to fetal and perinatal life but also extend to childhood, adolescence and adulthood. Macrosomic IMGDs generally remain overweight or obese during childhood and adolescence and are at high risk of hypertension, diabetes mellitus and metabolic syndrome during young adulthood, and of ischemic heart disease and atherosclerosis by middle age.[1,2]
Since most predictors of fetal macrosomia are modifiable, identifying them facilitates primary prevention of this GD complication and its associated adverse maternal and perinatal outcomes. Cuban studies on the subject are scarce, and FAC percentile assessment is not a part of routine obstetric practice in Cuba. Hence, this research aims to demonstrate the usefulness of this method for predicting fetal macrosomia in IMGDs, studying a group of Cuban women with GD and the value of the two curves used in Cuba to determine fetal weight percentile (Campbell and Wilkin[9] and Usher and McLean[10]).
The research hypothesis was that fetal macrosomia in IMGDs can be predicted by certain maternal risk factors (initial overweight or obesity, diagnosis of GD at >30 weeks, excess weight gain, inadequate glycemic control, hypertriglyceridemia and hypercholesterolemia) and fetal conditions (FAC >75th percentile and weight >90th percentile for gestational age at >28 weeks). Therefore, the objective of this study was to identify which of these maternal (clinical and laboratory) and fetal (US) variables are predictors of fetal macrosomia in IMGDs.
METHODS
Type of study and participants A retrospective case–control study was carried out, based on administrative data, between October 2002 and December 2012 (inclusive) in the antenatal diabetes service of the América Arias University Maternity Hospital (HUGAA) in Havana, Cuba. The universe comprised 1243 women with GD who gave birth at this hospital during the study period (approximately 3.9% of all births during the period), and who resided in HUGAA catchment municipalities (Centro Habana, Habana Vieja, Cerro and Habana del Este).
Sample calculation Sample size calculation was based on the assumption of 70% prevalence of overweight or obesity at pregnancy onset in cases and 46% in controls.[11] We specified 90% power to detect an odds ratio of 2.74 with α < 5%. The estimated sample size needed was 90 patients each in case and control groups. Taking into account a 5% probability of nonresponse (related to missing data in clinical records), the final sample size was 96 per group. However, since that number did not differ greatly from the number of women with GD who had macrosomic infants in the study period, we decided to include all of them in the study. Hence the sample size was 118 in each group.
Selection of cases and controls Cases were all women with GD who gave birth to a live macrosomic infant during the study period. Controls were women with GD who gave birth to a live nonmacrosomic infant, each the next delivery following that of one of the cases. Women whose infants were twins or underweight were excluded from the controls.
Variables Continuous variables included initial body mass index (BMI, kg/m2), gestational age at GD diagnosis (weeks), total pregnancy weight gain (kg), average monitored blood glucose (mmol/L–mg/dL), fasting plasma triglycerides (TG, mmol/L, single determination), fasting plasma total cholesterol (mmol/L, single determination), third-trimester FAC (mm), and fetal weight determined by FAC (g). All variables, except weight by FAC, were categorized to create qualitative or categorical variables (Table 1).
Data collection Information was extracted from medical records of women with GD treated at HUGAA during the study period, which also included data from primary health care records.
Techniques and procedures The following procedures reflect HUGAA protocol during the study period (in present tense) and authors’ analytic strategies (in past tense).
Initial nutritional assessment of pregnant women with GD uses BMI and the criteria of the Institute of Nutrition and Food Hygiene (INHA).[12]
GD diagnosis uses the National Comprehensive Diabetes Pregnancy Care Program criteria: ≥2 fasting blood glucose tests of ≥5.6 mmol/L (100 mg/mL) or blood glucose ≥7.8 mmol/L (140 mg/dL) in a 2-hour 75-g oral glucose tolerance test.[13] To define early GD diagnosis, we chose an upper limit of 30 weeks’ gestation, since it has been reported that GD must be diagnosed and therapy started before this gestational age to prevent macrosomia.[17]
Table 1: Variables

BMI: body mass index FAC: fetal abdominal circumference GD: gestational diabetes TG: triglycerides
Assessment of total pregnancy weight gain follows INHA criteria.[12]
Assessment of glycemic control is determined weekly with an electronic reflectometer (glucometer) (glucoDr, South Korea). To assess glycemic control, the last six weekly blood glucose levels were averaged; glycemic control was considered inadequate if mean monitored blood glucose was >5.0 mmol/L (90 mg/dL (criterion of National Comprehensive Diabetes Pregnancy Care Program).[13]
Lipid assessment is considered normal if maximum fasting plasma TG of <3.39 mmol/L during the third trimester of pregnancy and total cholesterol <6.60 mmol/L, both obtained from a single determination.[14]
FAC percentile assessment is determined by US in the third trimester of pregnancy, using an Aloka SSD 1100 (Japan) apparatus, with a 5 MHz transducer. The Tamura and Sabbagha curve[16] was applied to assess whether FAC was elevated, with the 75th percentile chosen as the upper limit of normal.[8]
Calculation of fetal weight by FAC in the third trimester used the Campbell and Wilkin curve.[9]
Assessment of fetal weight percentile in the third trimester used both the Campbell and Wilkin[9] and the Usher and McLean[10] curves/tables of fetal weight percentiles for gestational age. The 90th percentile of fetal weight for gestational age was chosen in both cases as the upper limit above which fetal growth was considered excessive.[8]
Birth weight determined in newborns using a scale (ATOM, Japan). Macrosomia in the newborn was diagnosed according to the National Gynecology and Obstetrics Expert Group criterion: birth weight >4000 g.[15]
Data analysis and presentation Proportions (percentages) were estimated for analysis of qualitative variables; for quantitative ones, means and standard deviations (SD) were calculated. To determine differences between the cases and controls, the Pearson chi-square (X2) was used for qualitative variables and the student t test for quantitative. A statistical significance of p <0.05 was specified.
Odds ratios (OR) were estimated with the corresponding 95% confidence intervals (CI) to estimate the effect size of independent variables on the dependent variable (macrosomia diagnosed at birth). Results for continuous variables were presented in a two-way contingency table, and those for categorical variables in a table of means, SDs and p values.
Ethics Information obtained from medical records was kept confidential. The study was approved by HUGAA’s scientific council and the research ethics committee of the National Endocrinology Institute.
RESULTS
Statistically significant differences between cases and controls were observed in the means for six of eight continuous variables, exceptions being initial BMI and fasting plasma cholesterol (Table 2).
Among categorical variables, only differences in late GD diagnosis and hypercholesterolemia failed to reach significance. The highest ORs were for excess estimated fetal weight (>90th percentile per Usher and McLean OR 8.81, 95% CI 4.25–18.26; >90th percentile per Campbell and Wilkin, OR 4.75, 95% CI 1.42–15.84), elevated FAC (OR 7.54, 95% CI 4.04–14.06), hypertriglyceridemia (OR 4.80, 95% CI 2.34–9.84) and excess pregnancy weight gain (OR 3.10, 95% CI 1.72–5.57). Initial maternal overweight or obesity, and inadequate glycemic control also had OR >2 (Table 3).
Table 2: Macrosomia prediction, continuous variables
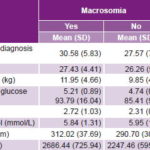
BMI: body mass index FAC: fetal abdominal circumference
GD: gestational diabetes TG: triglycerides
Table 3: Macrosomia prediction, categorical variables

FAC: fetal abdominal circumference GD: gestational diabetes
DISCUSSION
The results for late GD diagnosis do not concur with those of some authors. For example, Szymańska demonstrated that when GD diagnosis is made at 24–28 weeks, it is associated with decreased prevalence of macrosomia, compared to later diagnosys.[18] Torres estimated a relative risk (RR) of macrosomia of 2.123 at >32 weeks.[6] García found that 75.6% of women with a macrosomic newborn had GD diagnosed at >20 weeks;[19] Cruz found 84% had GD diagnosis at >30 weeks[11] and 77.5% at >32 weeks.[7] However, Dang found no statistically significant difference between women with and without a macrosomic infant, regarding GD diagnosis at <30 versus >30 or <32 versus >32 weeks, which agrees with our results.[20] The lack of statistical difference between cases and controls is likely due to the fact that in both groups, more than half were diagnosed with GD after 30 weeks’ gestation.[13] In Cuba, an oral glucose tolerance test for GD diagnosis is done at 28–32 weeks (rather than at 24–28 weeks, as in some countries), which explains why so many are diagnosed late by the 30-week criterion.
Initial overweight or obesity was associated with fetal macrosomia in IMGDs in our research. This finding was not unexpected, since obese women, even without GD, have more children with macrosomia than those who begin their pregnancy at normal weight.[21,22] Ouzounian[23] and Van Wootten and Turner[24] demonstrated that elevated BMI in early pregnancy is associated with macrosomia in IMGDs. Kerche[25] and Cypryk[26] reported that high BMI is a risk factor for macrosomia, with OR = 1.83 and OR = 2.38, respectively. Similar results have also been found in Cuban studies.[6,7,11,19,27]
We also found excessive weight gain during pregnancy associated with fetal macrosomia in IMGDs. This implies, at least theoretically, an excessive supply of nutrients to the fetus. This variable was a risk factor for IMGD macrosomia in studies by Tanir,[28] Ouzounian[23] and Kerche (OR = 1.79).[25] Cruz,[ 7] Park,[29] and Wong and Russell[30] also demonstrated that this variable is associated with neonatal macrosomia in the IMGDs.
Our findings indicated association between inadequate glycemic control and IMGD macrosomia. Hyperglycemia indicates excessive glucose supply to the fetus and fetal hyperinsulinism onset, directly responsible for IMGD macrosomia. This result is consistent with international[25,31] and national[7,11] studies that show poor glycemic control of GD associated with fetal macrosomia; however, this was not found by Lim.[32]
Hypertriglyceridemia was associated with IMGD fetal macrosomia. GD often induces dyslipidemia, characterized by a marked increase in TG and, consequently, free fatty acids, and very little or no plasma cholesterol. Several studies have shown that elevated maternal TG is associated with fetal macrosomia in IMGDs,[7,31,33] consistent with our results. This was not the case, however, in one study by Couch.[34]
Since maternal cholesterol during pregnancy serves predominantly to produce placental steroid hormones and not as a fetal nutrient, its association with macrosomia in IMGDs has been questioned, unlike TG. Our results for cholesterol are consistent with those of Cruz[7] and Whyte,[35] who found no association between maternal hypercholesterolemia and IMGD macrosomia.
FAC >75th percentile for gestational age at ≥28 weeks, was associated with fetal macrosomia in IMGDs, although the wide CI 95% range should be noted. FAC is the single US measurement most strongly correlated with birth weight and by far the most commonly used to determine fetal weight.[8,36] Schaefer-Graf reports that two US with FAC <90th percentile are enough to exclude the risk of IMGD macrosomia.[37] Schaefer-Graf,[38] Bochner,[39] and Tamura[40] found that FAC >90th percentile in the third trimester of pregnancy was significantly associated with fetal macrosomia in IMGDs. Kjos[41] reports a similar result, but with FAC >70th percentile.
In our study, fetal weight >90th percentile for gestational age ≥28 weeks, both by Campbell and Wilkin and by Usher and McLean curves, was associated with fetal macrosomia in IMGDs. This has been demonstrated in other studies.[7,18,39,42,43] Nelson found the association for a fetal weight >75th percentile.[43] García,[19] Tamura[40] and Wyse[44] for >90th percentile and Cruz for >97th percentile.[7] However, Vedavathi[45] found no correlation between FAC and fetal weight with IMGD birth weight, and Johnstone[46] reported that fetal weight was not a predictor for macrosomia, inconsistent with our results. The findings of Vedavathi[45] and Johnstone[46] could be explained by the small sample of women with GD included in their studies.
Regarding results for the continuous variables, we found no significant BMI difference between groups, but the opposite occurred when this variable was studied as qualitative. This could be the effect of continuous variables’ regression to the mean, which tends to dilute the observed effect. Dang and Gu, on the other hand, found statistically significant differences in initial BMI between groups of macrosomic and nonmacrosomic IMGDs (p = 0.000 and p = 0.008, respectively).[20,47] However, unlike us, Dang[20] found no statistically significant difference (p = 0.850) between the groups for mean blood glucose control.
Since macrosomia is the most frequent complication in IMGDs and the source of almost all their other complications (traumatic, respiratory, cardiovascular, metabolic, hematologic and septic disorders), preventing its onset implies improving maternal and perinatal outcomes for women with GD. An early GD diagnosis (before 30 weeks of pregnancy) is one of the first requirements for achieving this goal.
In Cuba, this responsibility rests mainly with doctors in primary health care, who must identify pregnant women at risk of GD. However, they should also be capable of identifying which women with GD are more likely to have macrosomic infants. This can only be achieved if they are well acquainted with conditions associated with macrosomia in IMGDs. Many of these conditions or determinants are sociocultural, such as poor nutrition, addictions, inadequate schooling, low socioeconomic status and unemployment.[48] Lack of consistent information on these conditions in patients’ medical records limited our ability to examine these associations. We do believe that primary care physicians are well positioned to pay close attention to these conditions, since theirs is a community-based practice, facilitating frequent personal contact with their patients. What’s more, Cuban women have an average of 16 antenatal visits per pregnancy.[49]
Our univariate analysis suggests that some maternal conditions are risk factors for IMGD macrosomia, but this needs verification by multivariate analysis, adjusting for different covariates. A limitation was our sample size calculation without access to prevalence data on some of the less frequent conditions in the underlying population (e.g., gestational age at GD diagnosis, hypercholesterolemia), which may have restricted the study’s ability to detect significance in the associations observed. Another limitation is not having taken into account newborns’ sex, which influences birth weight.
The novelty of this study lies in identifying a defined percentile value for FAC and fetal weight (applying two of the tables/curves used in Cuba) as predictors of neonatal macrosomia in IMGDs. The caveat is that these are international curves (there are no Cuban ones), so the source and application populations are of limited comparability. Professionals caring for women with GD at different levels in Cuba’s national health system should be aware of these specific percentile values of FAC and fetal weight, to intervene early to avoid macrosomia, the major complication in IMGDs.
CONCLUSIONS
Initial overweight or obesity in pregnancy, excess pregnancy weight gain, inadequate glycemic control, hypertriglyceridemia, and FAC >75th percentile and fetal weight >90th percentile for gestational age ≥28 weeks, were significantly associated with macrosomia in IMGDs and can therefore be considered predictors of this complication. We recommend instructing physicians caring for women with GD, especially in primary health care, to consistently assess these macrosomia risk factors or predictors, to help prevent the serious complications associated with this frequent growth disorder in IMGDs. We also recommend larger studies on this subject, in which not only clinical variables and laboratory tests are assessed, but also sociodemographic factors. At the same time, it becomes clear that Cuban FAC and fetal weight tables should be developed to increase the sensitivity of IMGD macrosomia diagnosis.






