INTRODUCTION
The global epidemic of chronic kidney disease (CKD) and consequent burgeoning demand for renal replacement therapy overwhelm health budgets of developing countries,[1] and only a few countries have economies robust enough to cope with growing CKD challenges.[2] The main traditional CKD causes reported worldwide are diabetes mellitus (DM) (responsible for 30%–40% of cases) and hypertension (HT) (25%–30%), mainly associated with cardiovascular risk factors and unhealthy lifestyles.[2–4] Glomerulonephritis and unknown causes are more frequent in Asia and sub-Saharan Africa than in the developed world.[5]
Environmental toxins known to increase CKD risk include chronic exposure to: heavy metals (lead, cadmium, arsenic, mercury and uranium);[6–9] agrochemicals;[6,10–12] nephrotoxic substances such as aristolochic acid, found in starfruit (Averrhoa carambola L., associated with Balkan endemic nephropathy);[5,6,13] and some Chinese medicinal plants.[14,15] Other CKD risk factors are chronic use of drugs such as NSAIDs, nephrotoxic antibiotics,[16] and sequelae of acute damage from poisoning, hypovolemia, obstruction or other causes.[17]
An increase in CKD incidence has been described in poor agricultural communities of Egypt, India and Sri Lanka.[8,11,18–21] Nontraditional risk factors, identified in several studies in these countries, are toxic, environmental and occupational. There appears to be a complex interaction between poor working conditions, notably inadequate handling of agrochemical mixtures—many of which are banned—used in large amounts and without protection; and prolonged, intense physical effort at high temperatures, with insufficient hydration.[22,23]
In Sri Lanka (2013), WHO recognized that CKD is associated with environmental contaminants, particularly heavy metals (cadmium and arsenic) contained in agricultural pesticides and fertilizers.[24–26]
In Central America and southern Mexico, an alarming CKD increase has been noted in the last two decades.[22,23,27–32] Epidemiological studies have found a high prevalence in agricultural areas, predominantly in men, mainly aged <60 years, exposed to agrochemicals in combination with other risk factors.[28–31]
Several efforts have been made in the region to characterize CKD that is not associated with traditional risk factors. Two etiologic hypotheses—both multifactorial but emphasizing different primary triggers—have been posited: one related to heat stress with repeated episodes of rhabdomyolysis and dehydration;[27,32] the other related to toxic exposures at work and in the environment of agricultural communities, coupled with presence of the aforementioned factors (harmful by themselves), which potentiate effects of prolonged, intensive use of agrochemicals.[29]
CKD constitutes a serious health problem in El Salvador. The Ministry of Health’s (MINSAL, the Spanish acronym) annual report for 2011–2012 stated that chronic renal failure (CRF) was the third cause of hospital death in adults: the first in men and fifth in women, with a case fatality of 12.6%.[33] In the 1990s, what appeared to be a different kind of CKD began to emerge in Salvadoran agricultural communities.[29–31] However, knowledge is incomplete concerning CKD frequency and distribution in the general population, and also about the clinical, physiopathological, anatomopathological and toxicoepidemiological characteristics of the CKD of nontraditional etiology in Salvadoran rural communities.
These knowledge gaps motivated this study, NefroSalva Epidemiológico. Objectives were to determine prevalence of CKD, CKD risk factors (traditional and nontraditional) and renal damage markers in the adult population of specific rural areas in El Salvador; measure population distribution of renal function; and identify associated risk factors in CKD patients detected.
METHODS
NefroSalva was carried out by the Renal Health Research Unit of MINSAL’s National Health Institute, supported by FOSALUD (El Salvador’s Health Solidarity Fund, based on tax revenue from tobacco, alcohol and firearms). The research team included Salvadoran doctors, nurses, clinical laboratory technicians, epidemiologists, community health promoters and nephrologists, with collaboration by students from the University of El Salvador Faculty of Medicine and Cuba’s Latin American Medical School, and active participation by community health committees in the areas studied. Experts from Cuba’s Nephrology Institute served as PAHO advisors to the study.
An analytical cross-sectional study was carried out in 2009–2011, combining epidemiological and clinical elements, including active screening for CKD cases and risk factors in the population aged >18 years in three impoverished regions of El Salvador: Bajo Lempa (Usulután Department), Guayapa Abajo (Ahuachapán Department) and Las Brisas (San Miguel Department).
Agricultural communities studied Bajo Lempa is a rural region on the banks of the Lempa River in eastern El Salvador,[34] an area of elevated CKD prevalence where communities are mainly dependent on agriculture.[29,31] Guayapa Abajo is an agricultural region (primarily sugarcane) in western El Salvador, with no previous epidemiological studies of CKD. Las Brisas in eastern El Salvador comprises suburban communities around San Miguel (capital of the department of the same name), and is the site of a former storage depot for toxaphene, known to have severe toxic effects on the kidney, liver and nervous system (banned by USEPA since 1990).[35] The abandoned depot was dismantled by Las Brisas residents. According to El Salvador’s Ministry of Environment and Natural Resources, toxaphene residues were found in nine of ten wells tested in 2009.[36]
Our study was carried out in two phases:
- Active screening for CKD, risk factors, renal damage markers in urine, and estimation of kidney function in the population
- Three months later, confirmation of renal damage markers in urine, stratification by CKD stages and analysis of association with risk factors in CKD patients
Study universe and population A door-to-door census identified 5018 persons (1306 families) in 11 communities studied in the 3 regions. Included were 2388 permanent residents aged >18 years in the 11 communities (976 men, 1412 women).
Study variables Displayed in Table 1.
Procedures Registration and coding Each patient was assigned a registration code for subsequent clinical monitoring.
Clinical history and physical examination were done to obtain personal information, personal and family medical history, and occupational and behavioral risks; plus physical measurements (weight, height, blood pressure, waist circumference).
Laboratory analyses A first morning urine sample was analyzed with reactive strips and strip-reading equipment, Multistix®10 SG Reagent Strip for Urinalysis (Bayer, USA). A 10-mL fasting venous blood sample was drawn to measure creatinine, glucose, cholesterol and triglycerides. Samples were processed in a laboratory installed in each region and equipped with a Cobas C111 spectrophotometer (Roche, Germany) and corresponding reagents.
Quality control, procedure standardization and data validation All measuring instruments and tools were calibrated to ensure data quality and consistency. Laboratory analyses were performed per manufacturers’ specifications using appropriate controls. Measurements and analyses were done by trained and certified personnel.
Data analysis Data were stored using Microsoft Excel for later export to SPSS 11.5 for Windows. Prevalence (%) was obtained for CKD, CRF and remaining study variables.
Contingency tables were created for bivariate analysis to explore CKD risk factors and eliminate those exceeding a significance threshold (p < 0.05). The independence test, Bartholomew test and chi-square test for regression were used, depending on whether the variable was nominal, ordinal or quantitative. To avoid collinearity in the multiple regression model, associations were first assessed using the chi-square test for qualitative variable independence with a correlation coefficient (phi or Cramer’s V, depending on whether the tables used 1 df or >1 df, respectively). The Pearson correlation coefficient (p) was used to assess associations for quantitative variables, a correlation >0.8 considered significant.
Later, logistic regression was applied to binary response variables to determine probability of developing CKD related to each risk factor, as well as simultaneous estimation of the pure or absolute influence of each factor on CKD or CRF risk while controlling for the others. The Enter method was used (which introduces all variables at the same time, instead of step by step). Variables whose coefficients were significantly different from 0 (p < 0.05), were identified using the Wald Test. Finally, the prevalence odds ratio (POR) was estimated for each variable and by intervals for each variable selected.
Model goodness of fit was evaluated using the Hosmer-Lemeshow chi-square test. The model was considered to fit if the probability of association with the statistical test was <0.05.
Ethical considerations Written informed consent was obtained from all participants, who agreed with publication of their results, ensuring confidentiality. All patients received clinical followup by health services.
Table 1: Study variables
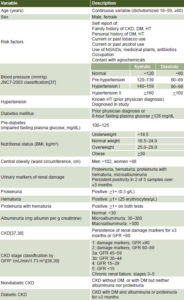
BMI: Body mass index CKD: chronic kidney disease DM: diabetes mellitus
GFR: glomerular filtration rate HT: hypertension
*calculated by MDRD formula[39] on mean of 2 creatinine levels measured ≥3 months apart by enzymatic method (SIEMENS, USA)
RESULTS
CKD-related risk factor prevalences in the study population are displayed in Table 2. Only 14.8% of the population were aged ≥60 years. Family history of CKD was found in 16.5%, of DM in 18.5% and of HT in 30.6%.
Alcohol and tobacco use were more common in men (27.2% and 18.6%, respectively) than in women (6.7% and 3.9%, respectively). Use of NSAIDs was similar in both sexes (84% in men, 84.3% in women), likewise of medicinal plants (50.5% in men, 52.3% in women). Starfruit consumption was not reported.
Men were more likely than women to be agricultural workers (54.3% vs. 15.2%) and to have contact with agrochemicals (66.5% vs. 33.1%) (Table 2). Eleven products were responsible for most direct agrochemical exposures: most frequently, organophosphate insecticides (methyl parathion, methamidophos, phoxim), bipyridylium herbicides (paraquat) and phenoxyacetic herbicides (2,4-D, hedonal).
HT was found in 17.7% of men and 23% of women. DM was not significantly different between men and women (8.5% vs. 9.3%). High prevalence of overweight was reported (28.6% in men, 28% in women). Obesity and central obesity were more frequent in women (26.1% and 30.7%, respectively) than in men (16.1% and 16.5%, respectively) (Table 2).
Renal damage markers and CKD prevalence are displayed in Table 3. These markers were found in 12.5% of the study population, more frequently in men than in women (16.3% vs. 9.8%). The most frequent marker was microalbuminuria (6.9%). Some 5.6% of the sample had glomerular filtration rate (GFR) <60 mL/mL/min/1.73 m2 body surface area with no renal damage markers. GFR steadily decreased with age and was lower in men of all age groups.
CKD prevalence was 18% (431/2388 persons); prevalence in men (23.9%, 234/976) almost double that of women (13.9%, 197/1412). Overall CRF prevalence was 11% (263/2388): 17.1% (167/976) in men and 6.8% (96/1412) in women, for a prevalence ratio of 2.5 for male sex (Table 3).
In the population aged 18–59 years, CKD prevalence was 14.1% (17.6% in men, 11.8% in women). CKD prevalence in those aged ≥60 years was 40.7% (57% in men, 27.6% in women) (Table 3). The largest number of CKD cases was in the younger age group in all three communities.
CKD prevalence in people working in agriculture at the time of the study was 26.8% (31.3% in men and 15.8% in women).
More than half the population in Bajo Lempa and Guayapa Abajo communities worked in agriculture (51.9% and 72.1%, respectively), but only 13.5% did so in Las Brisas, the area of highest CKD (21.1%) prevalence, followed by Guayapa Abajo (20.5%) and Bajo Lempa (15.4%). In Guayapa Abajo and Bajo Lempa communities, CKD prevalence in men was more than twice that in women; while in Las Brisas, prevalence was similar, 21.5% in women and 20.2% in men (Table 3).
Table 2: Prevalence of CKD risk factors in adults of Salvadoran agricultural communities, 2009–2011

an = 252 bn = 329 cn = 581
CKD: chronic kidney disease DM: diabetes mellitus HT: hypertension
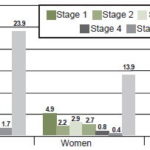
In general, prevalence decreases by CKD stage, but with an upward “bulge” from stage 2 to 3 (Figure 1). Most CKD patients (51.9%) had no DM, HT or proteinuria.
In the Bajo Lempa communities, male sex, age ≥60 years, agricultural occupation, family history of HT, and HT were significantly associated with CKD in univariate analysis. The logistic model revealed that likelihood of developing CKD was strongly influenced by male sex, aging, HT and agricultural occupation. CKD risk increased 4.5 times for age ≥60 years versus 18–59 years (POR 4.5, 95% CI 2.861–6.932). Agricultural activity doubled CKD risk compared to other occupations (POR 2.4, 95% CI 1.504–3.682); male sex almost doubled CKD probability (POR 1.9, 95% CI 1.145–2.442), as did HT (POR 1.8, 95% CI 1.198–2.753) and having a first-degree relative with HT (POR 1.9, 95% CI 1.139–2.364).
In Guayapa Abajo communities, age ≥60 years and agricultural occupation were significantly associated with CKD in univariate analysis. The logistic model showed that for age ≥60 years almost quadrupled CKD risk (POR 3.7, 95% CI 2.012–6.880), while agricultural occupation tripled CKD risk (POR 3.1, 95% CI 1.470–6.505).
In Las Brisas communities, age ≥60, family history or presence of HT, family history of CKD, tobacco use, family history of DM, and contact with the agrochemicals methyl parathion and methomyl were significantly associated with CKD in univariate analysis. The logistic model revealed that exposure to methyl parathion increased CKD probability by 2.6 times (POR 2.6, 95% CI 1.239–5.448).
Table 3: Prevalence of renal damage markers and CKD in Salvadoran agricultural communities, 2009–2011

CKD: chronic kidney disease CRF: chronic renal failure GFR: glomerular filtration rate
* Occupation not recorded for 44 Las Brisas residents
DISCUSSION
Our results show a clear vulnerability in these poor agricultural communities to increased risk of CKD development, progression and complications. This is consistent with research on low socioeconomic status and CKD severity in 1657 patients in the United Kingdom, which demonstrated that patients with lower economic status had greater risk of decreased GFR, after adjusting for other sociodemographic, behavioral, and clinical variables.[40]
The substantial prevalences we observed of family history of CKD, DM and HT could suggest increased genetic susceptibility to these conditions and/or predisposing environmental factors.They were similar to prevalences reported in other studies. For example, the KEEP-Japan study reported prevalence of family history of these conditions in the general population as 1.2%; 32.7% and 58.7%, respectively.[41] In Latin America, the KEEP-Mexico and KEEP-Jalisco studies reported a 52% prevalence of family history of CKD, and 23% prevalence DM and HT together.[42]
We found higher population prevalence of DM than reported in most studies from other countries: USA (1999–2004) 10.3% and (2008) 7%,[39,43] Spain 9.2%,[44] Cuba 5.4%,[45] and Mexico 8%.[46] HT prevalence in this study was lower or similar to that seen elsewhere: USA (1999–2004) 29%,[43] Spain 24.1%;[44] Cuba 30%;[45] and Mexico 7.7%–10% and 31%.[42,46]
Prevalence of current or previous tobacco use was close to that found in the Nefrolempa 2009[29] study and in a rural community study in Mexico.[47] Prevalence of alcohol use was high in men, recalling the hypothesis from a Nicaragua study linking CKD with a homemade alcohol (known as lija).[48] Our study did not determine the most frequently consumed types of alcohol.
The prevalence we found of overweight and obesity (the latter particularly in women) is consistent with international rates reported,[40,42–45] and indicates both need and scope for primary prevention, since obesity is associated with vascular and renal damage.
Figure 1: CKD prevalence by age and sex in Salvadoran agricultural communities, 2009–2011 (n = 2388, 976 men, 1412 women)
The high prevalence of pesticide exposure in our study is not surprising. A 2007–2008 study on pesticide contamination in Bajo Lempa detected dieldrin in shrimp culture ponds at concentrations of 0.085–0.182 ng/mL, 1.5 times higher than USEPA limits. Dieldrin was used for cotton crops until its agricultural use was banned in El Salvador in 1986. Six of the ten pesticides reported in our study were found in shrimp culture ponds: heptachloride, endrin, dieldrin and DDT metabolites—DDD and DDE. All of these are known to persist in soil as well.[49]
In Nicaragua, increased CKD rates in farmers aged <60 years were associated with pesticide exposure, dehydration, alcohol consumption, exposure to heavy metals and living at low altitudes.[48] Studies in Sri Lanka have found association between bipyridylium (paraquat and diquat) and organophosphate poisoning with repeated episodes of acute renal failure.[10]
In a recent study, Jayasumana described a chronic kidney disease of uncertain etiology affecting Sri Lankan agricultural communities, with predominance in men and onset in adolescence. He examined 125 patients and found skin hyperpigmentation on palms of the hands and soles of the feet; and toxicological studies demonstrated arsenic in biological samples. He concluded that the disease represented a new type of nephropathy, probably caused by arsenic poisoning with contributions by heavy metals and pesticide residues, and potentiated by heat stress, chronic repeated dehydration, poor quality drinking water and genetic susceptibility.[18] Also in Sri Lanka, Athuraliya detected histopathological findings demonstrating tubulointerstitial nephritis with or without nonspecific interstitial lymphocytic infiltration associated with glomerular atrophy and glomerular loss.[19] CKD in Sri Lanka is characterized by tubular proteinuria—usually alpha 1 and beta 2 microglobulin and elevated tubular proteinuria due to NGAL (>300 ng/mg creatinine/dL)—without edema.[19]
In our study, the relative absence of urinary renal damage markers, even in many patients in advanced CKD stages, strengthens the possibility of primary tubulointerstitial damage rather than glomerular impairment. Together with the upward surge in prevalence at stage 3a, this suggests possible substantial underdetection of stages 1 and 2, in persons with CKD who cannot be diagnosed because screening at early stages depends on renal damage markers. If this is the case, there is an even larger CKD iceberg looming beneath the surface.
CKD prevalence in both sexes was higher than rates commonly reported internationally,[41–45,50–53] but CKD was twice as frequent in men as in women from the earlier stages. The exception was Las Brisas, a community exposed to an agrochemical warehouse. Higher CKD prevalence in men than women in our study in part reflects the fact that men had high prevalences of both traditional and nontraditional risk factors for vascular and renal damage, the latter including poor working conditions, and contact with agrochemicals—some of which are highly toxic and/or banned by international conventions—without adequate protection. Only in Las Brisas was CKD prevalence similar in the two sexes, and it was the only community where men did not vastly outnumber women as farmworkers.
Reports on the CKD pandemic emphasize the increased burden on health systems with rising numbers of cases because of population aging, since risk increases with age.[2,3] However, in all three communities, the absolute number of CKD cases was higher in the group aged 18–59 years than in those aged ≥60 years: Such early onset and high CKD frequency in the economically active population has disastrous consequences for communities.
In our study, multiple logistic regression did not demonstrate statistically significant association of CKD with DM in any of the areas studied, contrary to the KEEP-Japan study, which reported a statistically significant POR of 1.71 for DM; KEEP-Japan’s statistically significant POR of 3.42 for HT was similar to our finding.[41]
HT prevalence is higher in patients with CKD and varies inversely with GFR; it is both a risk factor for cardiovascular morbidity and mortality and a progression factor for CKD. Some 80%–85% of CKD patients develop HT.[41] In our study, HT prevalence was higher in CKD patients, but the cross-sectional study design precludes establishing temporality, a limitation for interpreting the results.
Most CKD cases were not accompanied by DM, HT, or nephrotic proteinuria. CKD-associated factors revealed, according to the logistic model, that the possibility of developing CKD was strongly influenced by male sex, advanced age, agricultural occupation, HT, family history of HT and contact with the agrochemical methyl parathion. This reinforces the proposal of a new CKD with a different epidemiological pattern than hitherto predominating in most of the world, and that corresponds to the nephropathy described in agricultural communities of other Central American countries and Sri Lanka.[25,27–32,48] Accumulating evidence for the hypothesis of nephrotoxic environmental and occupational factors supports the need for more thoroughgoing investigation.
Among the study’s limitations is the equation used to estimate kidney function; the MDRD formula is the most widely used in epidemiologic studies,[38,39] but underestimates GFR in young adults and overestimates it in elderly patients. This should be considered when analyzing age-specific results. Also, the equation was designed for the US white and Afro-American populations and requires validation for the Salvadoran population.
Nevertheless, the study design is useful to estimate population prevalence, identify possible risk factors, and to generate research hypotheses. Its flexibility for studying associations between multiple exposures and multiple effects compensates for its limitations for assessing causation.
Finally, the information obtained has been useful for planning programs to meet the health care needs of the affected population. It was the basis for the primary care prevention component in MINSAL’s health reform. Comprehensive care has been established in the regions studied. In Bajo Lempa, a multidisciplinary team has been trained to provide prevention and treatment. Methodological and practical lessons from the study have been extended to other areas and have facilitated new health screening and interventions in other rural communities of El Salvador.
CONCLUSIONS
The results of this study reinforce the hypothesis emerging from other research suggesting a new nephropathy, which could be called agricultural nephropathy.
- Barsoum RS. Chronic Kidney Disease in the developing world. New Engl J Med [Internet]. 2006 Mar 9 [cited 2013 Jun 15];354(10):997–9. Available from: http://www.nejm.org/doi/full/10.1056/NEJMp058318
- El Nahas AM, Bello AK. Chronic kidney disease: the global challenge. Lancet [Internet]. 2005 [cited 2013 Sep 2];365(9456):331–40. Available from: http://www.sciencedirect.com/science/article/pii/S0140673605177897
- Collins AJ, Foley RN, Herzog C, Chavers B, Gilbertson D, Herzog C, et al. US Renal Data System 2012 Annual Data Report. Am J Kidney Dis [Internet]. 2013 Jan [cited 2013 Sep 6];61(1 Suppl 1):A7, e1–476. Available from: http://www.ajkd.org/article/S0272-6386(12)01404-7/fulltext
- Shaw C, Pruthi R, Pitcher D, Fogarty D. UK Renal Registry 15th Annual Report: Chapter 2 UK RRT Prevalence in 2011: National and Centre-Specific Analyses. Nephron Clin Pract [Internet]. 2013 Jan [cited 2013 Sep 6];123 Suppl 1:29–54. Available from: http://www.karger.com/Article/Pdf/353321
- Jha V, Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al. Chronic kidney disease: global dimension and perspectives. Lancet [Internet]. 2013 Jul 20 [cited 2013 Aug 15];382(9888):260–72. Available from: http://www.sciencedirect.com/science/article/pii/S014067361360687X
- Soderland P, Lovekar S, Weiner DE, Brooks DR, Kaufman JS. Chronic kidney disease associated with environmental toxins and exposures. Adv Chronic Kidney Dis [Internet]. 2010 May 17 [cited 2013 Sep 3];17(3):254–64. Available from: http://dx.doi.org/10.1053/j.ackd.2010.03.011
- Edwards JR, Prozialeck WC. Cadmium, diabetes and chronic kidney disease. Toxicol Appl Pharmacol [Internet]. 2009 Aug 1 [cited 2012 Dec 4];238(3):289–93. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2709710&tool=pmcentrez&rendertype=abstract
- Gonick HC. Nephrotoxicity of cadmium & lead. Indian J Med Res [Internet]. 2008 Oct;128(4):335–52. Available from: http://icmr.nic.in/ijmr/2008/october/1001.pdf
- Sabath E, Robles ML. Medio ambiente y riñón: nefrotoxicidad por metales pesados [Renal health and the environment: heavy metal nephrotoxicity]. Rev Nefrol [Internet]. 2012 [cited 2013 Sep 3];32(3):279–86. Available from: http://scielo.isciii.es/pdf/nefrologia/v32n3/revision_corta1.pdf. Spanish.
- Bandara JMRS, Senevirathna DMAN, Dasnayake DMRSB, Herath V, Bandara JMRP, Abeysekara T, et al. Chronic renal failure among farm families in cascade irrigation systems in Sri Lanka associated with elevated dietary cadmium levels in rice and freshwater fish (Tilapia). Environ Geochem Health [Internet]. 2008 Oct [cited 2013 Jan 7];30(5):465–78. Available from: http://www.springerlink.com/index/j418v18044554571.pdf
- El Minshawy O. End-stage renal disease in the El-Minia Governorate, upper Egypt: An epidemiological study. Saudi J Kidney Dis Transplantation. 2011 Sep;22 (5):1048–54.
- Mostafalou S, Abdollahi M. Pesticides and human chronic diseases: evidences, mechanisms, and perspectives. Toxicol Appl Pharmacol [Internet]. 2013 Apr 15 [cited 2013 Aug 29];268(2):157–77. Available from: http://ac.els-cdn.com/S0041008X13000549/1-s2.0-S0041008X13000549-main.pdf?_tid=f99905fa-baae-11e3-b340-00000aab0f6c&acdnat=1396474822_a40a562c708a4b520f99c685d75c4326
- Stefanović V, Polenaković M. Fifty years of research in Balkan endemic nephropathy: where are we now? Nephron Clin Pract [Internet]. 2009 [cited 2013 Sep 7];51–6. Available from: http://www.karger.com/Article/FullText/213081
- Jha V. Herbal medicines and chronic kidney disease. Nephrology (Carlton) [Internet]. 2010 Jun [cited 2013 Sep 3];15 Suppl 2:10–7. Available from: http://onlinelibrary.wiley.com/doi/10.1111/j.1440-1797.2010.01305.x/full
- De Broe ME. Chinese herbs nephropathy and Balkan endemic nephropathy: toward a single entity, aristolochic acid nephropathy. Kidney Int [Internet]. 2012 Mar [cited 2013 Sep 3];81(6):513–5. Available from: http://dx.doi.org/10.1038/ki.2011.428
- Hoitsma AJ, Wetzels JF, Koene RA. Drug-Induced nephrotoxicity. Aetiology, clinical features and management. Drug Saf [Internet]. 1991 Mar–Apr [cited 2013 Sep 8];6(2):131–47. Available from: http://link.springer.com/10.2165/00002018-199106020-00004
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012;2(1):S1–138.
- Jayasumana MACS, Paranagama PA, Amarasinghe MD, Wijewardane KMRC, Dahanayake KS, Fonseka SI, et al. Possible link of chronic arsenic toxicity with Chronic Kidney Disease of unknown etiology in Sri Lanka. J Nat Sci Res [Internet]. 2013 [cited 2013 Sep 3];3(1):64–73. Available from: http://www.iiste.org/Journals/index.php/JNSR/article/view/4193
- Athuraliya NT, Abeysekera TD, Amarasinghe PH, Kumarasiri R, Bandara P, Karunaratne U, et al. Uncertain etiologies of proteinuric-chronic kidney disease in rural Sri Lanka. Kidney Int [Internet]. 2011 Dec;80(11):1212–21. Available from: http://dx.doi.org/10.1038/ki.2011.258
- Wanigasuriya KP, Peiris-John RJ, Wickremasinghe R. Chronic kidney disease of unknown aetiology in Sri Lanka: is cadmium a likely cause? BMC Nephrol [Internet]. 2011 Jul [cited 2013 Aug 27];12:32. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3143923&tool=pmcentrez&rendertype=abstract
- Woo KT, Choong HL, Tan HB, Chin YM, Chan CM. On uncertain etiologies of proteinuric-chronic kidney disease in rural Sri Lanka. Kidney Int [Internet]. 2012 Jun [cited 2013 Aug 20];81(12):1277. Available from: http://dx.doi.org/10.1038/ki.2012.56
- El Salvador National Institute of Health. Declaración de San Salvador – Abordaje integral de la enfermedad renal túbulo-intersticial crónica de Centroamérica (ERTCC) que afecta predominantemente a las comunidades agrícolas [Internet]. San Salvador: Ministry of Public Health and Social Assistance of El Salvador; 2013 [cited 2013 Sep 8]. 6 p. Available from: http://www.salud.gob.sv/novedades/noticias/noticias-ciudadanosas/235-abril-2013/1820–26-04-2013-declaracion-de-san-salvador-abordaje-integral-de-la-enfermedad-renal-tubulo-intersticial-cronica-de-centroamerica-ertcc-que-afecta-predominantemente-a-las-comunidades-agricolas.html. Spanish.
- Pan American Health Organization. Chronic Kidney Disease in Agricultural Communities in Central America. Washington, DC: Pan American Health Organization; 2013. p. 20.
- World Health Organization. Chronic Kidney Disease of Unknown Aetiology (CKDu). A New Threat to Health. Sri Lanka: World Health Organization; 2013. p. 2.
- Mendis S. Mission Report. Chronic Kidney Disease of Uncertain Aetiology (CKDu), Sri Lanka. Geneva: World Health Organization; 2011. p. 2.
- Jayasumana C, Gunatilake S, Senanayake P. Glyphosate, hard water and nephrotoxic metals: are they the culprits behind the epidemic of chronic kidney disease of unknown etiology in Sri Lanka? Int J Environ Res Public Health [Internet]. 2014 Feb [cited 2014 Mar 21];11(2):2125–47. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3945589/
- Brooks DR, Ramirez O, Amador JJ. CKD in Central America: a hot issue. Am J Kidney Dis. 2012 Apr;59(4):481–4.
- Cerdas M. Chronic kidney disease in Costa Rica. Kidney Int Suppl [Internet]. 2005 Aug [cited 2014 Mar 21];(97):S31–3. Available from: http://www.nature.com/ki/journal/v68/n97s/full/4496413a.html
- Orantes CM, Herrera R, Almaguer M, Brizuela EG, Hernández CE, Bayarre H, et al. Chronic kidney disease and associated risk factors in the Bajo Lempa region of El Salvador: Nefrolempa study, 2009. MEDICC Rev. 2011 Oct;13(4):14–22.
- Trabanino R, Aguilar R. Nefropatía terminal en pacientes de un hospital de referencia en El Salvador. Rev Panam Salud Publica [Internet]. 2002 [cited 2013 Sep 3];12(3):202–6. Available from: http://www.scielosp.org/pdf/rpsp/v12n3/12875.pdf. Spanish.
- García R, Domínguez J, Jansà JM, Oliver A. [Proteinuria and chronic renal failure in the coast of El Salvador: detection with low cost methods and associated factors]. Nefrología [Internet]. 2005 Jan [cited 2013 Aug 29];25(1):31–8. Available from: http://www.revistanefrologia.com/revistas/P1-E239/P1-E239-S132-A3144.pdf. Spanish, English.
- Peraza S, Wesseling C, Aragón A, Leiva R, García RA, Torres C, et al. Decreased kidney function among agricultural workers in El Salvador. Am J Kidney Dis [Internet]. 2012 Apr [cited 2013 Aug 24];59(4):531–40. Available from: http://www.sciencedirect.com/science/article/pii/S0272638611017859
- Ministry of Public Health and Social Assistance of El Salvador. Informe de Labores 2012-2013 [Internet]. San Salvador: Ministry of Public Health and Social Assistance of El Salvador; 2013. p. 212. Available from: http://www.salud.gob.sv/servicios/descargas/documentos/func-startdown/746/. Spanish.
- Hernández W. Nacimiento y Desarrollo del río Lempa [Internet]. San Salvador: SNET; 2005. p. 14. Available from: http://www.snet.gob.sv/Geologia/NacimientoEvolucionRLempa.pdf. Spanish.
- United States Environmental Protection Agency, Office of Prevention, Pesticides and Toxic Substances. Protect yourself from pesticides: guide for pesticides handlers. Washington DC: United States Environmental Protection Agency; 2006 Jun. 109 p.
- Ministry of the Environment and Natural Resources [Internet]. San Salvador: Ministry of Environment and Natural Resources (SV); [updated 2011 Jan 3]. Las huellas del Toxafeno; 2010 Dec 17 [cited 2013 Dec 12]. Available from: http://www.marn.gob.sv/index.php?option=com_content&view=article&catid=162:especiales&id=700:las-huellas-del-toxafeno-. Spanish.
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003 May 21;289(19):2560–72.
- Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011 Jul;80(1):17–28.
- Bakris GL, McCullough PA, Collins AJ. Executive summary: Kidney Early Evaluation Program (KEEP) 2008 annual data report. Am J Kidney Dis [Internet]. 2009 Apr [cited 2013 Oct 31];53(4 Suppl 4):S1–2. Available from: http://www.ajkd.org/article/S0272-6386(09)00034-1/fulltext
- Bello AK, Peters J, Rigby J, Rahman AA, El Nahas M. Socioeconomic status and chronic kidney disease at presentation to a renal service in the United Kingdom. Clin J Am Soc Nephrol [Internet]. 2008 Sep [cited 2013 Oct 31];3(5):1316–23. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2518794&tool=pmcentrez&rendertype=abstract
- Takahashi S, Okada K, Yanai M. The Kidney Early Evaluation Program (KEEP) of Japan: results from the initial screening period. Kidney Int Suppl [Internet]. 2010 Mar [cited 2013 Aug 27];77(116):S17–23. Available from: http://www.nature.com/ki/journal/v77/n116s/full/ki2009539a.html
- Obrador GT, García G, Villa AR, Rubilar X, Olvera N, Ferreira E, et al. Prevalence of chronic kidney disease in the Kidney Early Evaluation Program (KEEP) México and comparison with KEEP US. Kidney Int Suppl [Internet]. 2010 Mar [cited 2013 Aug 27];77(116):S2–8. Available from: http://www.nature.com/ki/journal/v77/n116s/full/ki2009540a.html
- Center for Disease Control and Prevention (CDC). Prevalence of chronic kidney disease and associated risk factors–United States, 1999-2004. MMWR Morb Mortal Wkly Rep [Internet]. 2007 Mar 2 [cited 2013 Oct 31];56(8):161–5. Available from: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5608a2.htm
- Otero A, de Francisco A, Gayoso P, García F; EPIRCE Study Group. Prevalence of chronic renal disease in Spain: Results of the EPIRCE study. Nefrologia [Internet]. 2010 [cited 2013 Sep 3];30(1):78–86. Available from: http://dialnet.unirioja.es/servlet/articulo?codigo=3361355
- Valdez RH, López MA. Estudio epidemiológico en la comunidad de la enfermedad renal crónica, enfermedad cardiocerebrovascular, hipertensión arterial y diabetes mellitus. Estudio ISYS, Isla de la Juventud. Havana: Editorial Ciencias Médicas; 2008. p. 41–9. Spanish.
- Velázquez O, Peralta M, Lara A, Pastelín G, Tapia R; Grupo ENSA 2000. Hipertensión arterial en México: Resultados de la Encuesta Nacional de Salud (ENSA) 2000. Arch Cardiol Mex [Internet]. 2002 Jan–Mar [cited 2013 Oct 31];72(1):71–84. Available from: http://new.medigraphic.com/cgi-bin/resumenMain.cgi?IDARTICULO=4293. Spanish.
- Guerrero JF, Rodríguez M. Prevalencia de la hypertension arterial y factores asociados en la población rural marginada. [Prevalence and risk factors related to systemic arterial hypertension in a rural marginated population]. Salud Publica Mex [Internet]. 1998 Jul–Aug [cited 2013 Oct 31];40(4):339–46. Available from: http://www.scielosp.org/scielo.php?script=sci_arttext&pid=S0036-36341998000400006&lng=en&nrm=iso. Spanish.
- O’Donnell JK, Tobey M, Weiner DE, Stevens L, Johnson S, Stringham P, et al. Prevalence of and risk factors for chronic kidney disease in rural Nicaragua. Nephrol Dial Transplant. 2011 Sep;26(9):2798–805.
- de López NA, Dolores M, Lozano R. Determinación de la contaminación por plaguicidas en agua, suelo, sedimento y camarones en los cantones Salinas del Potrero y Salinas de Sisiguayo en la Bahía de Jiquilisco [Internet]. San Salvador: Americas New Initiative Fund (SV); 2008 Dec. p. 11. Available from: http://www.uca.edu.sv/investigacion/documentos/DocumentoUCA_FIAES.pdf. Spanish.
- Zhang QL, Rothenbacher D. Prevalence of chronic kidney disease in population-based studies: systematic review. BMC Public Health [Internet]. 2008 Apr 11 [cited 2013 Aug 10];8:117. Available from: http://www.biomedcentral.com/1471-2458/8/117
- Hallan SI, Coresh J, Astor BC, Asberg A, Powe NR, Romundstad S, et al. International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. J Am Soc Nephrol. 2006 Aug [cited 2013 Oct 31];17(8):2275–84. Available from: http://jasn.asnjournals.org/content/17/8/2275.long
- de Lusignan S, Chan T, Stevens P, O’Donoghue D, Hague N, Dzregah B, et al. Identifying patients with chronic kidney disease from general practice computer records. Fam Pract [Internet]. 2005 Jun [cited 2013 Oct 31];22(3):234–41. Available from: http://fampra.oxfordjournals.org/content/22/3/234.long
- Mathew TH, Corso O, Ludlow M, Boyle A, Cass A, Chadban SJ, et al. Screening for chronic kidney disease in Australia: a pilot study in the community and workplace. Kidney Int Suppl. 2010 Mar;(116):S9–16.
THE AUTHORS
Carlos M. Orantes Navarro (Corresponding author: doktorantes@gmail.com), nephrologist. Renal health research coordinator, National Health Institute, Ministry of Health (MINSAL), San Salvador, El Salvador.
Raúl Herrera Valdés, nephrologist. PAHO advisor. Full professor and distinguished researcher, Nephrology Institute, Havana, Cuba.
Miguel Almaguer López, nephrologist. PAHO advisor. Associate professor and distinguished researcher, Nephrology Institute, Havana, Cuba.
Elsy G. Brizuela Díaz, physician, Metropolitan Health Region, San Salvador, El Salvador.
Lilian Núñez, physician. Director, Bajo Lempa’s Monseñor Romero Specialized Family Community Health Unit (UCSF-E), Usulután Department, El Salvador.
Nelly P. Alvarado Ascencio, physician. Western Health Region, MINSAL, Santa Ana, El Salvador.
E. Jackeline Fuentes de Morales, physician with a master of public health. Director, San Miguel UCSF-E, San Miguel, El Salvador.
Héctor D. Bayarre Vea, physician specializing in biostatistics. Full professor, National School of Public Health, Havana, Cuba.
Juan Carlos Amaya Medina, nephrologist, Bajo Lempa UCSF-E, Usulután, El Salvador.
Denis J. Calero Brizuela, nephrology resident, Nephrology Institute, Havana, Cuba.
Xavier F. Vela Parada, physician, Renal Health Research Unit, National Health Institute, MINSAL, San Salvador, El Salvador.
Susana M. Zelaya Quezada, physician, RHRU, National Health Institute, MINSAL, San Salvador, El Salvador.
Delmy V. Granados Castro, physician, RHRU, National Health Institute, MINSAL, San Salvador, El Salvador.
Patricia Orellana de Figueroa, laboratory technician. Coordinator of national clinical laboratory network, National Health Institute, MINSAL, San Salvador, El Salvador.







