Reprinted with permission from VacciMonitor (VacciMonitor, 16(3):13-19, September-December, 2007, http://www.finlay.sld.cu/publicaciones/vaccimonitor/Vm2007/a14.pdf.
ABSTRACT
Honduras was one of the Central American countries most severely hit by Hurricane Mitch. Torrential rains and heavy flooding created conditions conducive to a leptospirosis outbreak in the country. A group of Cuban scientists studied 68 patients from the Department of Cortés-one of the country’s hardest hit areas-presenting clinical and epidemiological profiles indicative of leptospirosis. Blood and serum samples were taken from all subjects. A microscopic agglutination test (MAT) was used to identify Leptospira strains and to assess protection conferred by vax-SPIRAL® (Cuban leptospirosis vaccine) against the isolated strain. Prevalence of leptospires in the kidneys and liver was also verified. A male predominance was found in the group aged 15-49 years. Municipalities in this Department with the largest number of cases were San Pedro Sula, La Lima, and Chamelecón. The most frequent symptoms included fever, headache, myalgia, and generalized discomfort. Over 80% of subjects reported presence of rodents in their homes, as well as contact with stagnant water and domestic animals. The strain isolated from positive blood cultures was from the Icterohaemorrhagiae serogroup, which was highly virulent in the animal model used. Protection was 100% in hamsters inoculated with vax-SPIRAL® and subsequently challenged with the Honduran strain. Additionally, macroscopic analysis of organs from immunized animals that survived the challenge showed no signs of leptospirosis infection.
Keywords: Leptospirosis, vaccine, serology, diagnosis, classification
INTRODUCTION
Leptospirosis is one of the most widely disseminated zoonoses in the world. It is considered a re-emerging disease with endemic and epidemic behavior. Its causative agent is a spirochete of the Leptospira interrogans species.[1] Leptospirosis may present as isolated cases, outbreaks, or epidemics, depending on exposure to a contaminated environment and the ability of surveillance systems to detect the disease.[2,3] Rodents and domestic animals are the main reservoirs of pathogenic leptospires, although some wild animals may also harbor these microorganisms. Human infection results from exposure to the urine of an infected animal, either by direct contact or through contaminated water. The impact of leptospirosis on morbidity and mortality in Central America is unknown, which may be due to inadequate recordkeeping, late case reporting, communication problems, insufficient laboratory service coverage, and limited response to outbreaks.[4]
Leptospirosis epidemics are frequent at certain times of year, mainly during the rainy season, since contaminated water is one of the primary sources of leptospire dissemination.[5]
Isolation of leptospires by serological testing is highly sensitive, constitutes de finitive proof of infection, and is also considered the reference method for evaluating other direct diagnostic tests. However, serology can be difficult due to sample contamination with other microorganisms and the slow growth rate of leptospires.[6-8]
In most Central American countries, few cases of leptospirosis are reported, the exception being Nicaragua, where leptospirosis has been a recognized public health problem since the 1995 epidemic, when about 2,000 cases and over 50 deaths were reported.[4] In 1998, constant rains from Hurricane Mitch caused severe flooding in Central America, leading to leptospirosis out-breaks in the region. One of the most severely affected countries was Honduras, particularly the Department of Cortés, where a group of Cuban scientists worked to detect and confirm a leptospirosis outbreak affecting humans and animals.
Given that leptospirosis constitutes an unresolved health problem in Central America, and climate changes prompt flooding followed by the spread of this zoonosis, we proposed to study an outbreak of leptospirosis in Honduras in the wake of Hurricane Mitch; verify the presence of leptospirosis and isolate the strain or strains causing the disease; and verify experimentally the protective immunity of the trivalent Cuban leptospirosis vaccine against the particular strain isolated in Honduran patients. Vaccination remains the most effective means of prevention if the appropriate vaccine is available.
METHODS
The study included 68 subjects with clinical and epidemiological profiles suggestive of leptospirosis, all from various localities in Health District III of the Department of Cortés, Honduras, an area that had recently been ravaged by Hurricane Mitch and suffered serious flood damage. This study was designed and conducted according to established ethical principles and standards for this type of research.
A data form specially designed for this study was completed for each subject suspected of having leptospirosis. Afterward, a 5-mL blood sample was taken from a peripheral vein in the forearm of each subject. Each sample was then deposited in two small sterile tubes with rubber stoppers and metal caps containing 5 mL of EMJH Leptospira culture medium.[9,10] The rubber stoppers of both tubes were punctured with the same needle used to draw blood, and 1 and 2 drops of blood were added to the tubes, respectively. The inoculated media were then incubated at 28 30°C, and the remaining blood was used to obtain sera through centrifugation for 10 minutes at 5,000 rpm. Finally, each tube was labeled and stored at -20°C until examination.
Cultures were examined every 5 days, and samples were taken from every tube showing signs of bacterial growth for subculture in enriched EMJH medium. Cultures were examined for 40 days and were not discarded until 45 days after inoculation. Subcultures of strains were made at least three times in EMJH medium under static growth conditions at 28-30°C to fulfill conditions for serologic classification.
Cultures were classified by a microscopic agglutination test (MAT), using reference polyclonal antisera specific for the Ballum, Pomona, Canicola, Icterohaemorrhagiae, Pyrogenes, Tarassovi, Australis, Hebdomadis, Batavie, Automalis and Sejroe serogroups, manufactured at the Leptospirosis Reference Laboratory of the Royal Tropical Institute (KIT), Netherlands, following the methodology described in the World Health Organization (WHO) Diagnosis, Surveillance and Control guidelines.[10] MAT analysis of sera was conducted using live antigens, and the respective reference homologous strains were added as agglutination controls for each antiserum [Mus 127 and Arborea (Ballum), 5621 (Pomona), Hond Utrecht IV (Canicola), M20 (Icterohaemorrhagiae), Salinen (Pyrogenes), Perepelicin (Tarassovi), Ballico (Australis), Hebdomadis (Hebdomadis), Van Tiene (Bataviae), Akiyami A (Autumnalis) and Hardjoprajitno (Sejroe)].
Virulence of isolates was qualitatively estimated in the Syrian golden hamster model ( Mesocricetus aureatus ), following the methodology described by González et al.[12] Groups of 5 animals weighing 45-50 g from the National Laboratory Animal Production Center (CENPALAB) were inoculated intraperitoneally (IP), and the following doses taken from a 5-6 day static culture in EMJH medium were used: 0.8 mL (60 x 10 7 cells), 0.4 mL (30 x 10 7 cells), 0.1 mL (7.5 x 10 7 cells), and 0.05 mL (3.5 x 10 7 cells) of a bacterial suspension adjusted at 7.5 x 10 7 cells/mL (direct cell count in a Petroff-Hausser chamber). After inoculation, the animals were observed for 14 days, and strains were classified as highly virulent when all hamsters died that had been given a bacterial suspension =0.1 mL. If the animals died after administration of a dose =0.4 mL, the strain was considered moderately virulent, and if mortality was <100%, strains were considered mildly virulent or avirulent. Current national and international bioethical standards and regulations were taken into account during the work with laboratory animals.
MAT analysis was used to evaluate the 68 sera from subjects suspected with leptospirosis. The methodology and interpretation of results are described in Human Leptospirosis: Guidance for Diagnosis, Surveillance and Control, published by the Expert Committee of the World Health Organization.[10] An initial 1:10 serum dilution was used.
Criteria for case interpretation:
Positivity Criteria. Subjects who met one of the following criteria were considered leptospirosis-positive:
- Isolation of leptospires from hemoculture.
- Significant MAT titer (=1:160) in serum sample.[13,14]
Individuals were considered negative when no leptospires were isolated in the hemoculture and no positive titers were obtained in the serum samples.
Reactivity criteria. Cases that met the following criterion were considered reactive:
- No significant MAT titers (=1:160) in serum sample.[13,14]
A case was conside red non-reactive for leptospirosis when no serological reactivity was obtained with the app lied test in a serum sample.
In each serum sample with a MAT titer =1:160, the serogroup showing the highest titer was presumed to be the infectious one, and when more than one serogroup had the same high titer, then the serogroup was regarded as undetermined.[11]
Evaluation of the protection conferred by vax-SPIRAL® against the challenge of the Honduran strain was conducted using 40 hamsters weighing 45-50 g from the National Laboratory Animal Production Center (CENPALAB). The animals were immunized with two intramuscular 0.5 mL doses of vax-SPIRAL® (batch 8011), administered at a 6-week interval, based on the schedule proposed by Naranjo et al.[16] Fourteen days after completing the schedule, the animals were challenged intraperitoneally with 10,000 LD 50 of each of the vaccine candidate strains ( L. canicola, L. icterohaemorrhagiae, L. pomona and the Honduran strain). Ten animals were used per strain, and the total inoculum volume was 0.05 mL. Forty animals that had not been immunized were used as controls.
The inoculated animals were observed for 14 days, and those that survived in each group were euthanized, and a macroscopic anatomopathological examination was performed. Liver and kidney cultures were also grown in EMJH medium to ascertain the prevalence of the microorganism in the main target organs for Leptospira infection.[17] Organ cultures were observed periodically under a dark-field microscope, and the absence of growth after a 60-day incubation period at 30°C under static culture conditions was considered a negative result.
RESULTS
Table 1 shows the demographic distribution of the 68 subjects studied. Of suspected cases of leptospirosis, 40 (58.8%) were men and 28 (41.2%) were women. There was a clear predominance of males and subjects aged 15-49 years, which coincides with the risk groups observed in Cuba.
Table 1: Demographic Distribution of Population Studied

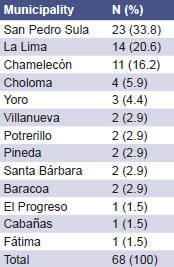
The three municipalities with the largest number of cases were San Pedro Sula, La Lima, and Chamelecón, in the Department of Cortés (Health District III) (Table 2).
Table 2: Distribution of Suspected Cases of Leptospirosis by Municipality of Residence
The most frequent symptoms were fever, headache, myalgia, general discomfort, calf pain, arthralgia, and abdominal pain (Table 3).
Fifty-five subjects (80.8%) reported presence of rodents in or around their homes, 59 (86.7%) reported contact with stagnant water, and 38 (55.8%) reported recent contact with domestic animals. The occupational distribution of the subjects studied was as follows: 20 farmers (29.4%); 19 housewives (27.9%); 8 students (11.7%); and 21 (30.8%) engaged in other activities.
Two hemocultures were positive (2.94%): the MAT serological classification for one corresponded to an Icterohaemorrhagiae serogroup strain with a titer of 1:10240, and the second could not be classified due to contamination of the culture (Table 4). Qualitative evaluation of the hamsters showed high virulence. There was no gradual increase in mortality in correlation with the dose administered (Figure 1).
Table 3: Clinical Signs and Symptoms in Suspected Cases of Leptospirosis

Table 4: Isolated Strain’s MAT Classification and Virulence Evaluation

MAT: Microscopic Agglutination Test HV: Highly virulent
Figure 1: Virulence of the L. icterohaemorrhagiae strain in the Syrian golden hamster animal model

Figure 2: Serogroups of leptospires diagnosed in serum samples from Honduras

MAT analysis for 44 of the patient serum samples (64.70%) showed no reactivity, while 24 (35.29%) were reactive; of these, 9 had titers >1:160 to at least one of the antigens used, and 15 had titers <1:160.
Presumed infectious serogroups were determined for the 24 MAT- reactive cases, as follows: 17 (44.4%) for L. icterohaemorrhagiae , 4 (22.2%) for L. hardjo , and 1 (11.1%) for L. canicola, L. pyrogenes , and L. tarassovi , respectively (Figure 2).
The only strain isolated corresponded to the Icterohaemorrhagiae serogroup.
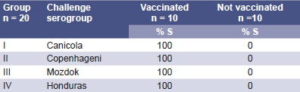
Results of protection attained in hamsters that were immunized with vax-SPIRAL® and challenged with the isolated strain are shown in Table 5. There was evidence of 100% protection in animals that were immunized and challenged with vaccine strains as well as with the Honduran strain, and of 100% mortality in the controls (animals not immunized).
Macroscopic analysis of org ans from immunized animals, 100 % of which survived th e cha llenge, showe d in all case s an absence of the ch aracteristic sign s of in fection (inflamma tio n, induration, hemorrhage or jaundice). The culture of several organ sections in EMJH medium revealed no microbial growth after 60 d ays of in cubation. Macroscopic analysis of organs of animals that were not immunized, however, showed all the characteristic signs of leptospirosis, and cultured sections of organs such as the liver and kidney showed growth of the Leptospira challenge strain as well as microscopic signs of the lesions caused by this strain.
Table 5: Results of Hamsters Vaccinated with vax-SPIRAL® Challenged with 10,000 LD 5 0 Virulent Vaccine Strains and the Honduran Strain
DISCUSSION
The climatic conditions brought about by Hurricane Mitch, combined with other factors, favored the re-emergence of many diseases, including leptospirosis, a disease rarely reported in Honduras due to poor epidemiological surveillance systems and problems detecting, investigating, and confirming leptospirosis cases, as well as lack of clinical and epidemiological knowledge about the disease. Furthermore, the existing laboratory network is not setup to confirm diagnoses of suspected leptospirosis cases.
Leptospirosis is clinically difficult to diagnose, as its causative agent may affect various organs, producing a great variety of clinical symptoms and errors in clinical diagnoses, such as influenza, aseptic meningitis, encephalitis, gastroenteritis, hepatitis, and dengue fever. Diagnosis of leptospirosis is essential and important for administering specific immediate therapy, thus contributing to a better clinical evolution of cases.[2,10,13,14]
Demographic characterization of the population in this study suspected of having leptospirosis is consistent with that described by other Cuban authors, who report a greater male prevalence of leptospirosis (80%), as well as a higher number of cases in the population aged 15-44 years (65.9%).[18] The most frequent anicteric symptoms among subjects in this outbreak were also consistent with those found by Cruz et al.,[18] who report the presence of fever (99.5%), headache (92.2%), myalgia (91.4%), and arthralgia (86.4%). Moreover, as with this study, the main source of infection was found among farm workers in wet terrain with a high mice infestation (39.7%). The municipalities with the most affected individuals are located in the most hurricane-ravaged and poorest areas, where the large numbers of rodents and domestic animals favor the spread of leptospirosis and human infection.
Laboratory confirmation of leptospirosis is challenging. Detection of agglutinating antibodies by different serological methods and isolation of strains are compromised by an indication for early and aggressive treatment, although the individual response of each patient influences immunoglobulin detection.[6,8,11,15,17,19] Behavior of the immune response to leptospirosis varies. An individual exposed to Leptospira strains for the first time develops a wide variety of signs and symptoms that may be linked to the virulence of the microorganism and its antigen load.[20] The conclusive test for serological diagnosis is the MAT, a method developed in the United States by the Centers for Disease Control and Prevention (CDC). This technique is time-consuming and laborious, and calls for experienced technicians and the maintenance of multiple live serovars.[6,10,15] Human infection may be caused by more than one circulating serogroup, and, given the characteristics of the MAT, a panel of antigens that are epidemiologically significant in the local area must be used.
On the other hand, strain isolation through hemoculture (the “gold standard”) is especially important for diagnosing leptospirosis. Although it does not provide early diagnosis of the infection, it can retrospectively confirm it and accurately define the responsible serovar.[2,21] Hemoculture has low sensitivity and requires a special medium and more than six weeks of incubation.[2,21] This study combined the two techniques in an effort to provide diagnosis and information with greater accuracy and future utility.
Serogroups observed in clinical cases examined in this study matched the results of most of those identified by the MAT in Central America, fundamentally the 1995 Nicaraguan outbreak strains.[22] The results obtained in Honduras, using a combination of serological and bacteriological techniques, together with clinical and epidemiological diagnoses, permitted confirmation or definition of probable cases among subjects who were evaluated on suspicion of leptospirosis infection.
Whole cell leptospirosis vaccines provide effective protection against fatal infection in immunized animals, even though such protection is short-term and restricted to the serovars in the vaccine and to antigenically related strains.[23] While evaluating the efficacy of a vaccine against these microorganisms, however, a distinction must be made between protection against fatal infection and protection against emergence of a carrier condition (organ infection and leptospiruria). The absence of leptospirosis in immunized animals does not preclude the possibility that they are asymptomatic carriers capable of disseminating leptospires through urine.[17] Whereas the carrier condition in humans is short-lived after natural infection and is not usually a major source of leptospirosis dissemination, in the case of animals (cattle or pigs), chronic asymptomatic carriers may persist, thereby acting as major agents of infection.[17] Accordingly, a good prophylactic leptospirosis vaccine is required for effective protection against fatal infection and the carrier condition.
In this study, macroscopic analysis of organs obtained from animals immunized with vax-SPIRAL® and which survived the challenge revealed the absence of the characteristic signs of infection. The culture of several sections of those organs in EMJH medium showed no microbial growth after 60 days of incubation. Full protection against the carrier condition provided by vax-SPIRAL® coincides with that obtained after administering other adjuvant leptospirosis vaccines,[23] and cellular immune response enhanced by aluminum hydroxide apparently plays a key role.
Studies conducted in Cuba following immunization of different risk groups with vax-SPIRAL® have demonstrated a 60.4% decrease in total cases and even point to cross-protection against serovars of leptospires not included in the vaccine.[25] This good broad-spectrum protector response may be explained by the ability of this type of formulation with particulated antigens and additional adjuvation to induce an immune response with a Th-1 type pattern.[24] Based on the information above, the deci sion was made to use the Syrian golden hamster model to test protection provided by vax-SPIRAL® against the most widely disseminated strain in the Honduran outbreak, a test that produced satisfactory results. Taking both experiences together, it can be established that vax-SPIRAL® , widely used in Cuba, is a safe vaccine with minimal reactogenicity that may be considered a very useful prophylactic measure during flood situations similar to those that occurred in Honduras, as well a s in populations in high-risk areas. In the absence, to date, of a tailor-made leptospirosis vaccine manufactured with Central American strains, it can provide direct protection against the serovars included in its formulation (several of which coincide), and is also capable of inducing cross-protection against other serovars, as shown in previous studies.[26,27]
Subsequent studies confirmed an epidemic outbreak of 172 leptospirosis cases in Honduras, 28 of which were confirmed through laboratory tests and the rest reported by clinical and epidemiological diagnosis; seven of the cases were fatal.[28]
References

- Barthi AR, Jarlath EN, Ricaldi JN, Matthias MA, Díaz MM, Lovett MA, et al. Leptospirosis. A zoonotic disease a global importance. Lancet Infect Dis 2003;3(12):757-71.
- Levett P. Leptospirosis. Clin Microbiol Rev 2001;14(2):296-326.
- Suárez M, Morera J, Díaz C and Sánchez JM. Brotes de Leptospirosis animal y humana en la provincia de ciego de Avila Rev.Cub Med Trop 2005;57(1):79-80.
- OPS. Principales enfermedades infecciosas en Centroamérica durante 1998, antes y después del Mitch. Rev Panam Salud Pública 1999;6(6):1-30.
- Sejvar J, Bancroft E, Winthrop K, Bettinger J, Bajan M, Braga S, et al. Leptospirosis in “EcoChallenge” athletes, Malaysian Borneo, 2000. Emerg Infect Dis 2003;9(6):702-7.
- Terpstra WJ, Hartskeerl, RA, Smits, HL. and Korver, H. International Course in Laboratory Techniques for the Diagnosis of Leptospirosis. Royal Tropical Institute, Amsterdam, 2000.
- Rodríguez J, Rodríguez JE and Fernández C. Alcalinización de la orina humana para el aislamiento experimental de Leptospiras. Rev. Cub Med Trop 2005,57(1): 55-6.
- Viretz JM. Leptospirosis. Curr Opin Inf Dic 2001;14:527-38.
- Ellinghausen H, McCullough W. Nutrition of Leptospira pomona and growth of 13 other serotype: a serum-free medium employing oleic albumin complex. Am J Vet Res 1965;28: 39-44.
- WHO-ILS. Human leptospirosis: Guidance for diagnosis, surveillance and control. Malta: WHO; 2003.
- Hartskeerl RA, Smits HL, Korver H, Goris MGA, Terpstra WJ, Fernández C, et al. International course on laboratory methods for the diagnosis of leptospirosis. Havana: Palcograf; 2006.
- González A, Betistov, Véldes and González M. Crecimiento, virulencia y antigenicidad de Leptospira interrogans Server mozolata en medio EMJH modificado. Rev. Cub Med Trop 2002;54(1):32-6.
- Ministerio de Salud Pública. Programa Nacional de Prevención y Control de la leptospirosis humana. Cuba: MINSAP; 1998.
- Ministerio de Salud Pública. Programa Nacional de Prevención y Control de la leptospirosis humana. Cuba: MINSAP; 2000(updated version).
- Effler PV, Domen HY, Bragg SL, Aye T, Sasaki DM. Evaluation of the Indirect Hemagglutination Assay for Diagnosis of Acute Leptospirosis in Hawaii. J Clin Microbiol 2000;38(3):1081-4.
- Naranjo M, Rodríguez Y, Oliva R, Jáuregui U, González M. Esquema de inmunización en hámsters frente al preparado vacunal antileptospirósico cubano. Acta Fram Bonaerense 1999;18:121-6.
- Faine S, Adler B, Bolin C, Perolat P. Leptospira and leptospirosis. 2nd ed Melbourne: Edit Me- diSci; 1999.
- Cruz R et al. Prevención y Control de la Leptospirosis humana. Presented at International Leptospirosis Conference; 2006, Havana.
- Obregón AM, Fernández C, Rodríguez I, Rodríguez J, Fernández N, Enrique G. Importancia de la confirmación microbiológica en un brote de leptospirosis humana en la ciudad de Villa Clara. Rev Cub Med Trop 2003;55(2):96-9.
- Bernasovskaia EP, Kondratenko VN, Melnitskaia EV. The connection of the antigenic activity of Leptospira to its virulence. Mikrobiol. Z. 1994;56(6):46-50.
- Palmer MF, Zochowski WJ. Sulvival of leptospirosis in commercial blood cultive systems revisited. J Clin Pathol 2000;53:713-4.
- Trevejo RT, Rigau-Perez JG, Ashford DA, et al. Epidemic leptospirosis associated with pulmonary hemorrhage-Nicaragua 1995. J Infect Dis 1998;178:1457-63.
- Bolin C, Alt D. Use of a monovalent leptospiral vaccine to prevent renal colonization and urinary shedding in cattle exposured to Leptospira borgpetersenii serovar Hardjo. Am J Vet Res 2001;62:995-1000.
- Brown R, Blumerman S, Gay C, Bolin C, Duby R, Baldwin C. Comparison of three different leptpospiral vaccines for induction of a type 1 immune response to Leptospira borgpetersenii serovar Hardjo. Vaccine 2003;21:4448-58.
- Martínez R, Pérez A, Quiñones M, Cruz R, Álvarez A, Armesto M, et al. Eficacia y seguridad de una vacuna contra la leptospirosis humana en Cuba. Rev Panam Salud Pública 2004;15:249-55.
- González A, Rodríguez Y, Batista N, Valdés Y, Núñez JF, Mirabal M, González M. Inmunogenicidad y capacidad protectora en hámsters de vacunas antileptospirósicas monovalentes de células enteras del serogrupo Ballum. Rev Argentina Microbiol 2005;37:169-75.
- González M, Batista N, Machado M, Savournin O, Saltarén A, Sanamñe A, et al. Caracterización de cepas de Leptospira Ballum aisladas de casos clínicos. Inmunidad cruzada en hámsters vacunados con vax-SPIRAL® . Biotecnología Aplicada 2004;21:77-81.
- Secretaría de Salud de Honduras. Programa de Preparativos para Desastres, OPS/OMS. Serie Crónicas de Desastres. Huracán Mitch en Honduras 1998. Tegucigalpa M.D. C. Febrero de 1999.
VacciMonitor Editors’ Note: This article, originally published in the print edition of VacciMonitor (2007;16(3):13-19), has been updated by request of the authors for this online version.
THE AUTHORS
Gustavo Sierra (Corresponding Author: gsierra@finlay.edu.cu), Mariela Naranjo, Marta González, Niurka Batista, Irma González, Yolanda Valdés and Juan F. Infante, Finlay Institute Vaccine Research and Production Center, Havana, Cuba.
Miguel Suárez, Provincial Hygiene and Epidemiology Center, Ciego de Ávila, Cuba.
Carmen Fernández, Pedro Kourí Institute of Tropical Medicine, Havana, Cuba.
Nelly Amador, Health Department, San Pedro Sula, Honduras.
Erratum
González García S, Fernández Concepción O, Fernández Carriera R, Menéndez Sainz C, Maza J, González-Quevedo Monteagudo A, Buergo Zuaznábar MA. Association between blood lipids and types of stroke. MEDICC Review. 2008;10(2):27-32.
Page 28, full paragraph 8, second sentence should read: “Serum was stored at -20°C for no longer than 20 days.”









