It was just before New Year’s Eve, 2019 when an emerging virus in China caught the attention of Dr Ileana Morales, director of Science and Technological Innovation in Cuba’s Ministry of Public Health. She had already participated in implementing Cuban protocols to prevent Ebola and address diseases such as Zika and dengue. But this was different, and COVID-19 would prove the biggest challenge in her decades-long career in public health leadership. As she put it: a “trial by fire.” Over two years later, in March 2022, she spoke with MEDICC Review providing a firsthand account of the Cuban strategies, results and lessons learned in the nation’s colossal efforts to stem the tide of a pandemic that still threatens the world.
MEDICC Review: After that glimpse of the virus detected in China, what were the first steps you and the Ministry took?
Ileana Morales: From that start, we began gathering all the information available internationally, and then as a Ministry and a government, we got to work planning. We knew we needed a coordinated national plan involving everybody, from the community level to the various ministries, which would be used across the country. So, every ministry was asked to revise their protocols for addressing epidemics, including first and foremost, the Ministry of Public Health (MINSAP). By January 30, 2020, the first version of the National Plan to Address COVID-19 was approved at the highest levels. [The first cases of COVID-19 were diagnosed in Cuba on March 11, 2020.–Eds.][1]
MEDICC Review: And that plan began to change your life, and that of many others…
Ileana Morales: It certainly did. At that point, the Plan emphasized prevention, surveillance—these were at the heart of our Ministry’s efforts. But my life really changed on February 12, when the National Science Group was created to confront the coming epidemic…that was perhaps the first big step, and the Group was co-chaired by myself and Dr Rolando Pérez, who heads research at BioCubaFarma, our biotech conglomerate. But the group had a multisector design from the start, and we began bringing in experts from every field…public health, biotech, social sciences, animal health, you name it. There were about 15 of us at that point, and we began meeting right away, and we’ve met daily ever since, to this day.
MEDICC Review: What have been the Science Group’s main responsibilities over the course of the pandemic?
Ileana Morales: We had three main tasks, which later evolved of course. At the start, it was to keep abreast of the available information on the disease throughout the world, monitor how it was behaving, what was new. From there, we created a COVID-19 Observatory online where we posted everything available globally. Second, we were tasked with finding out what was available in Cuba itself to address such a virus: epidemiological research, biotech products that might be repurposed, or others that were in development. Third, we delved into health system studies that could help us better organize our response. The whole idea was—and is— to put science to work. Science became the engine for our whole pandemic response.
MEDICC Review: The Ministry of Public Health has had a fundamental role in the process, and in the creation of the National Protocols as well, correct?
Ileana Morales: Of course. The first national treatment protocol, step-by-step guidance, was created on February 16th. At that point, it was basically a list of what might be used to treat the disease, and a few days later we included the steps to be taken for epidemiological monitoring. All the proposals were put on the table for the Science Group to consider, and on February 26th, the Innovation Committee was formed, also chaired by Dr Pérez and myself. Included in the Innovation Committee were other key actors from research and manufacturing centers, as well as the directors of the national regulatory authority and of the clinical trials coordinating center. These last two became doubly important when we began developing our own vaccines.
Thus, we had the Science Group making recommendations, and the Innovation Committee choosing what aspects, products or strategies to introduce, in coordination with government. Together, final decisions were made. The Innovation Committee also approved all versions of the national protocols applied across the country, which were revised periodically based on our own experience in Cuba and those of other countries. That is, these were not set in stone, but periodically revised, informed by the results as we continued to study COVID-19.
The idea is that there isn’t a single medical decision taken that isn’t supported by a study, a trial or an approved therapy.
We began to include other important elements regarding not only prevention but also diagnosis and of course refining the treatments needed according to severity of symptoms, comorbidities, etc. The idea was to get this information to our health professionals as fast as possible, so they would have the latest and best guidance, whether at the community level or a tertiary care facility. The idea is that there isn’t a single medical decision taken that isn’t supported by a study, a trial or an approved therapy. Today’s protocol has over 200 pages!
Each new protocol version was presented to the National Temporary Technical Group, chaired by President Miguel Díaz-Canel, for final authorization. And by April 2020, both the Science Group and the Innovation Committee were meeting weekly with the President. The aim was to generate evidence-based decisions throughout the pandemic.
MEDICC Review: Several Cuban biotech products have been introduced into the treatment protocol over the last two years. How was this done?
Ileana Morales: I remember early in 2020, one of our biotech experts brought to the table the idea of using Cuban interferon to treat some patients, and so right there, we decided to launch a clinical study to determine its potential, a study approved by the clinical trials coordinating center and the regulatory authority. And then there were other products, already in use, but which had to be evaluated specifically for treating COVID-19. This was true, for example with some that had anti-inflammatory potential to curb the cytokine storm. Each one had to be tested, studied and the results assessed amidst the rising tide of the epidemic. There was no time to lose, but we also couldn’t afford to make superficial decisions. People’s lives were at risk. People were counting on us.
MEDICC Review: Nevertheless, the country was under other pressures, especially economic ones, with the US embargo still in effect, the collapse of tourism, and the needs of the health system itself. With the decision to re-open airports in November 2020, the number of COVID-19 cases began to surge. In this context, how were the epidemiological priorities considered?
Ileana Morales: Virtually every decision passed through the lens of science. At that point in particular, we were looking at Cuba’s rating on the Oxford COVID-19 Government Response Tracker (Oxford index), how we were doing compared to other countries internationally.[2] We had a rating of nearly 100, the highest on the scale in terms of stringency of measures. So, in November and December 2020, we were seeing good epidemic control, and with that in mind, the Science Group experts began to analyze how to open the country. Our epidemiologists and mathematicians recommended opening because of the low incidence of cases we had managed to achieve at that point. But what happened was that in January 2021, the delta variant entered into play, which we hadn’t foreseen.
Delta was responsible for the great case surge we experienced from June through September in various provinces, such as Havana, Matanzas and Ciego de Ávila. Those months were very hard, but also full of lessons, especially for the clinical protocols and the medicines to be used, who were the most vulnerable patients, how to work with children and with the elderly, and so on.
MEDICC Review: Amidst all this, the government decided to develop Cuban vaccines. How did this come about? How did it interface with your own work?
Ileana Morales: In May 2020, President Díaz-Canel met with scientists at the Finlay Vaccine Institute, and they all concluded that developing Cuban vaccine candidates offered the best hope of being able to vaccinate our whole population. And so, the biotech centers, including Finlay and the Genetic Engineering and Biotechnology Center, began their research, based on their decades of experience developing and producing vaccines.
Thus began the challenge of pre-clinical research and later the clinical trials, all of which had to be presented first to the Science Group and thereafter to our national regulatory authority for approval—the design, certification of trial sites, data collection, everything. And finally, the Science Group had to address vaccination rollout: if and when we had efficacious vaccines authorized for emergency use, how were we going to distribute them? Who would get them first? How would we prepare health personnel, and certify vaccination centers in primary health care? Keep records and provide the necessary follow-up for adverse reactions?
MEDICC Review: How did the vaccine clinical trials and other studies evolve towards mass vaccination?
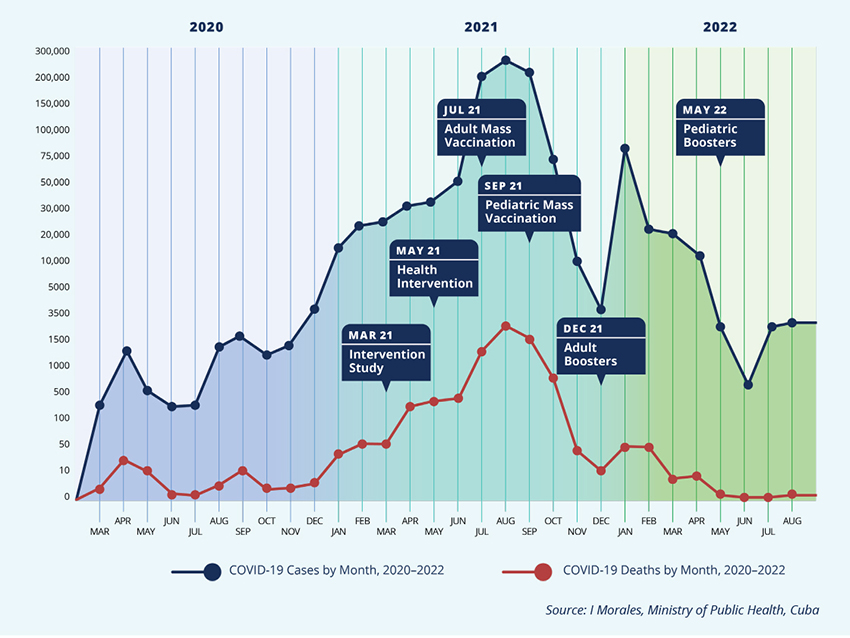
Ileana Morales: From mid-August 2020 to March 2022, 20 vaccine clinical trials have been conducted. Plus another 30 related to other products. And after preliminary results from phase 2 clinical trials for the SOBERANA and Abdala vaccines, we initiated an intervention study, involving mainly health workers as well as biotech scientists, who received either of the two vaccine regimens (see graphic). We mounted a study protocol, with external research ethics committees and evaluation through site visits and monitoring of results and adverse events.
This was followed by the public health intervention, involving an even larger number of people in territories where the surge was peaking. Again, this was a complicated process, since the vaccines were still in clinical trials, although we had excellent safety and efficacy results in hand from phase 2 trials. But this required a ministerial resolution, based on our Public Health Law, which gives the minister specific extraordinary authority in health emergencies. The Science Group provided a risk-benefit analysis to guide the decision, and thus it became the most complex public health intervention we have ever undertaken in Cuba. We began vaccinating adults in four Havana municipalities, the ones experiencing the greatest incidence, and later extended the process to other municipalities experiencing surges. And if we hadn’t done that, who knows how high the peak of cases would have reached!? Havana was able to resist the delta variant better because of the public health intervention.
Havana was able to resist the delta variant better because of the public health intervention.
MEDICC Review: And parallel to that, the phase 3 trials of both the Abdala and the SOBERANA vaccine regimens were continuing, correct?
Ileana Morales: Yes, and in July 2021, Abdala received emergency use authorization from our regulatory agency, and in August the SOBERANAs also received authorization, in both cases for adults 19 years of age and older. Thus, in July, the national vaccination rollout went forward. In summary: some 100,000 people participated in the clinical trials, about 160,000 in the intervention study, 2.5 million in the health intervention and 8 million in mass vaccination. Every step, all the procedures, regulatory processes, good clinical practice guides, logistics, data collection and follow-up, were previously submitted to the Innovation Committee. Our regulatory agency, the Center for State Control of Medicines and Medical Devices (CECMED), made thorough reviews of all these aspects, inspected clinical trial sites and vaccine manufacturing facilities, and assessed the vaccine dossiers. During the process, CECMED authorities met often with vaccine producers and the clinical trials’ principal investigators to gain clarity and determine any changes in study protocols. Finally, after considering the results of each successive study and clinical trial, CECMED made the final decisions on emergency use authorization.[3]
MEDICC Review: An extraordinary process. What has it meant for you, for your work schedule?
Ileana Morales: It was very, very tough. An 18-hour day on average. And the hardest part was the responsibility you carried with you every minute. We would start early in the morning and wouldn’t leave the ministry until 1:00 or 2:00 in the next morning, seven days a week. It has been like a war, where everyone was needed on the frontlines. And it’s been very emotional. Dr Francisco Durán, national head of epidemiology, and I would finish our daily video chat with the provinces at about midnight, and then he would drop me home. We left with a lump in our throats, often with heavy hearts. Feeling the weight of the responsibility, desperately wanting to do more. I sometimes felt like I was carrying a whole building on my back. But all of us felt that way, I’m sure.
At the same time, we had confidence because we had so many talented people on our side, so much organization brought to bear for the whole country–the schools, teachers, even tourism workers pitched in. Not to mention the hospital and primary care professionals, the students.
The whole country was involved. How could we not have confidence? But it was still hard.
MEDICC Review: What about omicron now?
Ileana Morales: Thus far, we haven’t seen a big rise in cases. For me, the explanation is that Cuba vaccinated virtually the whole population very quickly, and then boosted. For some time, Cuba has been among the first, if not the very first country, in terms of doses applied per 100 population. That includes the three-dose original schedule, plus boosters. Our vaccination rates are over 90%.
MEDICC Review: And children vaccinated?
Ileana Morales: The vaccination of youngsters from 2 to 18 years was crucial. That happened after emergency use authorization and was critically important. Children are carriers and can impact transmission in the general population, which is especially dangerous for elderly people at home. Since vaccinating, we haven’t had one child die from COVID-19, not one pregnant woman, either. For some time, we haven’t had children with severe disease. The only critical cases we are seeing now are in immunocompromised adults, people in terminal stages of other diseases, and elderly with four or five comorbidities.
MEDICC Review: What has Cuba done differently than some other countries?
Ileana Morales: Our strategy has been different from some others in at least four ways. First, it has been a single strategy, applied throughout the country. It is flexible, of course, but essentially the same wherever you are, the clinical guidelines the same, too. Second, it’s a protocol that begins in the community and ends in the community. Why? Because it’s like a staircase…beginning with prevention and diagnosis in the community, and finally with convalescent patients followed up in their communities. In both cases, the neighborhood family doctors and nurses are key since they are at the heart of community care.
Third, it is a protocol that covers all aspects of pandemic response: from preventive measures to therapies and rehabilitation, including mental health. It’s all there, and that is also why we have taken care to update it periodically with the latest experiences and information. We developed a stratification model to accompany it. Which leads me to the fourth difference: the protocol depends on getting ahead of the curve. That is, if we applied a medication at one time when patients had become critical, later we decided to apply it before that stage, to prevent such severe complications. Thus, this was a kind of staircase: the aim was to prevent illness, and if a person became ill, then to prevent serious disease, and if they became serious, then to prevent death, and finally, for convalescents to recover as quickly as possible.
MEDICC Review: Lessons learned?
Ileana Morales: Many. First, that evidence-based decision-making provides the best, surest guide to action. I think that’s a lesson learned by the entire society, the entire government, too. Second, that health is too important to leave entirely to the Ministry of Public Health. So, it made sense for everyone to be at the table who had something to contribute: mathematicians, physicists, sociologists, demographers. Multi- and transdisciplinary discussions were essential, and nobody was asking permission to talk with anybody else. If you needed to talk with the university rector, you simply called her, or with a government official at any level. And for some of the most renowned scientists, you had to see them going with me to the provinces, to the communities, to the primary health care level. The borders were simply erased, red tape cut. And that made for more streamlined, coordinated action…something we need going forward. We also learned that we could introduce earlier some measures or medicines with the required studies when the situation and the evidence warrant.
We ratified the importance of the links between biotechnology and public health, between the universities, the various fields of study and population health. The aim is for our cumulative knowledge to be at the service of people’s health, to be able to quickly introduce the results that science can offer. And that is the challenge.
Finally, we once again learned how important international cooperation is, scientific and public health collaboration, sharing of experiences. This is vital. We have approved about one thousand research studies about COVID-19, 300 of these national studies that could help inform the global scientific community. And we’re certain that such potential exists elsewhere as well. All these lessons we learned during an experience that was traumatic. We learned them during our ‘test of fire.’
We have approved about one thousand research studies about COVID-19, 300 of these national studies that could help inform the global scientific community.
MEDICC Review: And that trauma extended to your own family.
Ileana Morales: Yes, my grandson, the light of my life, contracted COVID-19 early in the pandemic. Then my daughter and my son-in-law. He was the most serious case in the family, but received several of the Cuban biotech medications, and thankfully recovered. Then I tested positive, too. But I have to say, all this was not extraordinary. In one way or another, all of us have someone close to us who contracted the disease.
MEDICC Review: What can you say about the role of women in the sciences in this colossal effort? Cuba has a record of being one of the countries with the greatest percentage of women in the sciences, and health sciences in particular.
Ileana Morales: Yes, that’s been the norm for some time. What is interesting now is the role of women leading in the sciences. Because you could see many women as researchers, health workers, teachers…but it’s quite another thing to see women leading. For example, women are leading 70% of the primary research projects approved by the Innovation Committee. What’s more, when I look over the report we made to parliament in 2021—Scientific Advances to Confront COVID—you see women in the majority, leading the efforts and the results in virtually every aspect. And that includes vaccine development as well as the vaccination rollout.
MEDICC Review: Anything else, going forward?
Science empowers, and it has empowered women.
Ileana Morales: I’m reminded that our meetings used to start with a standard Cuban saying: “gentleman, what more can we do?”. But of course, this just isn’t right. It has to be at least “ladies and gentlemen”! The day I write a book about all this, that just may be the title. Science empowers, and it has empowered women.

- Cuba’s COVID-19 Strategy: Main Actions through April 23, 2020. MEDICC Rev. 2020 Apr;22(2):50‒2. https://doi.org/10.37757/MR2020.V22.N2.14
- Hale T, Angrist N, Goldszmidt R, Kira B, Petherick A, Phillips T, et al. A global panel database of pandemic policies (Oxford COVID-19 Government Response Tracker). Nat Hum Behav. 2021;5:529‒38. https://doi.org/10.1038/s41562-021-01079-8
- Aguilar-Guerra TL, Fajardo-Díaz EM, Gorry C. Cuba’s National Regulatory Authority & COVID-19: Olga Lidia Jacobo-Casanueva MS. Director, Center for State Control of Medicines and Medical Devices (CECMED). MEDICC Rev. 2021 Juy–Oct;23(3–4):9‒14. https://doi.org/10.37757/MR2021.V23.N3.3