INTRODUCTION
Acute respiratory distress syndrome (ARDS) is a complex heterogeneous disorder that involves severe progressive refractory hypoxemia and has various causes. ARDS may be pulmonary (severe pneumonia, radiation pneumonitis, bronchial aspiration of stomach contents, pulmonary contusion, inhalation of toxic gases, oxygen toxicity) or extrapulmonary (sepsis, septic shock, nonpulmonary trauma, acute pancreatitis, pesticide poisoning, multiple transfusions of blood and blood products, and intracranial hypertension).[1] ARDS is an unresolved health problem associated with sepsis and multiple organ failure:[1] Nearly 50 years after Ashbaugh described it,[2] ARDS case fatality in children is still 30%–40%. Ventilation with positive end-expiratory pressure (PEEP) is the fundamental and most important treatment strategy.[1,3,4]
Exogenous surfactants have been used to treat ARDS in several clinical trials,[5–7] all of which reported better lung function, reflected in improved oxygenation. Willson found a significant reduction in mortality, although not in ventilation time, length of stay, or time in intensive care.[7] Although results are promising, surfactant use in children needs rigorous evaluation, since there is no consensus among pediatricians on treatment regimen dosage and frequency.[1,5] Surfactant effects are short-lived because of inhibition by plasma proteins, which has led to the idea that small, repeated doses could be more effective than a single dose. It is imperative that more clinical trials be conducted with exogenous surfactants.[1]
In 1995, Cuba’s State Regulatory Authority for Drugs Medical Equipment and Devices (CECMED) approved use of the natural porcine surfactant Surfacen, developed in Cuba. Since it received CECMED registration (0800), Surfacen has become part of standard medical treatment of hyaline membrane disease in preterm newborns in all neonatal intensive care units (NICU),[8–10] which has helped lower Cuba’s infant mortality rate due to this cause.[11]
Surfacen has pharmacologic and biophysical properties that may stop ARDS or at least mitigate the complex inflammatory oxidizing process involved in its pathophysiology.[12] In planning this clinical trial, we posited that adding Surfacen to conventional treatment with oxygenation and mechanical ventilation should increase efficacy in terms of oxygenation recovery and survival in children with ARDS.
METHODS
Study type, patients and sample A phase III, nationwide, multicenter clinical trial was conducted at five pediatric hospitals in four provinces in central and eastern Cuba: two in Santiago de Cuba (Dr Antonio María Béguez César Pediatric Hospital and Dr Juan de la Cruz Martínez Maceira Children’s Teaching Hospital), one in Holguín (Octavio de la Concepción y la Pedraja Provincial Pediatric Teaching Hospital), one in Camagüey (Dr Eduardo Agramonte Piña Provincial Pediatric Teaching Hospital) and another in Villa Clara (José Luis Miranda Provincial Pediatric Teaching Hospital). The trial was open-label and controlled, with randomization into two treatment groups (experimental and control), and was conducted from November 2009 through August 2013.
Sample size calculation We considered observed case fatality the main dependent outcome. Because ARDS is rare, we used a two-stage sequential design that called for stopping the study as early as possible to compare the two rates and test the hypothesis that expected case fatality would be higher in the control group. We set expected case fatality at 70% for the control group and 40% for the experimental group, based on Surfacen’s excellent biophysical properties and anti-inflammatory effects. Type I and type II errors were calculated in R and preset as a and b values not to exceed limits of 6% and 20%, respectively. Based on these calculations, we confirmed that 18 patients per group would be sufficient in both the first and second stages. This design allowed us to optimize the study so that it could be stopped in the first stage, should there be early evidence of a positive effect of Surfacen on survival. This was possible because the sample size per group was larger than planned (>18) at the time of the first cut, and the difference in observed response rates exceeded the boundary value. The sequential method’s flexibility[13–15] allows sample size cuts that do not necessarily coincide with planned sample size when there is evidence of an effect, as long as type one error is sequentially controlled.
A randomization list was created by hospital and blocks of four (random sequences of two symbols, with each symbol occurring twice), using a table of random numbers from a uniform probability density function in the interval (0,1), generated with Statistica 5.5. This method ensured totally random patient assignment to the groups. Treatment assignment was conducted using sealed envelopes, each containing a card identifying the assigned treatment, labeled with a patient code. This method ensured that clinical investigators did not know a given patient’s assigned treatment group until the decision to initiate treatment was made.
The sample comprised 42 children. For two of them, the only data available came from the initial assessment and the exit point. One had been assigned to the experimental group but died before treatment began; the other was assigned to the control group but no longer met all selection criteria, so assessments were terminated. However, they were counted as study patients, because no withdrawal criteria had been set; i.e., all available data were processed.
Inclusion criteria Patients were selected who were aged 1 month to 18 years, diagnosed with ARDS according to the criteria of the 1994 American-European Consensus Conference on ARDS,[16] and whose parents gave written consent for participation.
Exclusion criteria Patients with blood diseases, cancer, congenital cardiopathy with increased pulmonary flow or signs of pulmonary hypertension, or known hypersensitivity to Surfacen were excluded.
Treatment groups There were 20 patients assigned to the experimental group and 22 to the control group. Patients in the first group received conventional treatment plus Surfacen (see below), and those in the control group received only conventional treatment. In both groups, as oxygenation improved, controlled respiratory assistance levels were modified to prevent ventilator-induced injury.
Variables Demographic variables were age (grouped in years: <1, 1–5, 6–10, >10) and sex (female, male). Clinical outcomes were selected according to guidance issued by the European Medicines Evaluation Agency.[17] The main dependent outcome was patient vital status (alive or deceased) 28 days after randomization.
ARDS presentation Clinical forms were classified as pulmonary (pneumonia, bronchial aspiration, pulmonary contusion, inhalation of toxic gases, near-drowning, mechanical airway obstruction and others) and extrapulmonary (sepsis, septic shock, nonpulmonary trauma, pancreatitis, extracorporeal circulation or cardiopulmonary bypass, thromboembolism, fat embolism, drug overdose, congenital diaphragmatic hernia, burns, multiple transfusions, electrocution, anaphylaxis, typhoid fever, hypereosinophilic syndrome).
Gasometric and ventilatory variables PEEP was obtained from the preset value on the mechanical ventilator (normal value 2–5 cm H2O). Maximum PEEP was obtained from the preset value on the mechanical ventilator and stratified into values of 6–10 cm H2O, 11–15 cm H2O, 16–20 cm H2O and >20 cm H2O. Inspired oxygen fraction (FiO2) was also obtained from the preset value on the mechanical ventilator (normal <60%).
Arterial oxygen tension (PaO2) was obtained from arterial blood gas results (normal 95–100 mmHg). Arterial carbon dioxide tension (PaCO2) was also obtained from arterial blood gas testing (normal 35–45 mmHg).
Kirby index (PaO2/FiO2) is a direct indicator of clinical progress in ARDS. We defined clinical progress and patient responsiveness by values of ≥200.
Oxygenation index (OI) was calculated as mean airway pressure x FiO2 x (100/PaO2) (normal <5).
Static lung compliance (SLC) was calculated as (exhaled tidal volume/plateau pressure) minus PEEP (normal 1–2 mL/kg weight/cm H2O).
Transcutaneous oxygen saturation (SaO2) was obtained from finger pulse oximetry (normal 95%–100%).
Radiographic course was classified as improved, stable or worse, depending on the reduction, persistence or increase, respectively, in pulmonary lesions observed in chest radiography.
Hospital outcomes Mechanical ventilation time was defined as days between intubation at time of ARDS diagnosis and extubation. Length of stay in pediatric intensive care unit (PICU) was defined as days from date of admission with ARDS until discharge from PICU.
Control treatment The conventional ARDS treatment protocol was used in participating hospitals’ PICUs:
- pressure-controlled ventilation
- hypercapnia permitted up to 80 mmHg
- tidal volumes close to physiologic values for age and body weight (5–7 mL/kg) in patients with respiratory system compliance, and stable volumes of 3–6 mL/kg in patients with poor lung compliance
- inspiration: expiration ratio from 1:2 to 2:1
- pH >7.2
- PEEP above the lower inflection point of the pressure/volume curve by static maneuvers, and sufficient to keep PaO2 above 60 mmHg in arterial blood gas if PEEP value revealed falling pulse oximetry saturation or the static compliance curve showed signs of alveolar overcompliance compromising the patient’s hemodynamics, recommended measures were initiated until full stabilization was achieved.
- FiO2 high enough to ensure SaO2 of 88%–90%.[16]
Investigational treatment The surfactant used in this trial was Surfacen, a porcine pulmonary surfactant produced and sold by Cuba’s National Center for Agricultural Health in collaboration with the National Biopreparations Center. It is supplied as a sterile white lyophilized product in a 6R vial containing 50 mg total phospholipids. It is composed of phospholipids (95%), mainly dipalmitoylphosphatidylcholine; hydrophobic proteins (SP-B and SP-C, 1.5%); and other lipids (3.5%).[18] Its anti-inflammatory and antibacterial effects have been shown in both in vitro and in vivo models,[19,20] as have its excellent biophysical properties.[12] Toxicology studies have shown that it is nontoxic.[21] The safety of Surfacen has been proven, its safety profile similar to that of other internationally marketed surfactants. No adverse reactions to Surfacen have occurred in any clinical trials.[7,8,22,23]
This consisted of conventional treatment plus intratracheal instillation of 100 mg of Surfacen diluted in 4 mL of water for injection (25 mg/mL), every 8 hours for 3 days, until 9 doses were completed. This low dose was used because there is no consensus on optimal dose, and because using amounts in keeping with those prescribed for newborns would involve intrapulmonary administration of a fluid volume (120 mL for a child weighing 30 kg) that would flood the alveoli, negatively affect gas exchange, and obstruct the airway. In addition, there are indications that what characterizes patients with ARDS is a change in phospholipid profile, not in total amounts of phospholipids.[24] In a phase II clinical trial of Surfacen in adult ARDS patients, repeated doses of 100 mg produced a significant improvement in oxygenation.[25]
Radiographic monitoring After diagnosis of ARDS, chest x-rays were performed on the first three days and on the fifth day after enrollment. During daily rounds, PICU specialists assessed all x-rays to date as a set. Findings were then summarized by radiologists in written reports. Results were compared to initial x-rays to assess changes, if any, in amounts of inflammatory infiltrates in lung fields, to classify radiographic course.
Data collection and analysis All data were recorded in the study patients’ medical records and case report forms, and entered into a database for statistical processing. Available data were used for patients who died before completing all protocol assessments, so that some analyses did not include all patients who started the study.
Processing and statistical analysis used the significance threshold and power set during sample size determination (5% significance threshold and 81.7% power).
The main analysis of efficacy was a one-tailed test comparing the percentage of surviving patients in the two groups 28 days after enrollment. We chose a one-tailed test because of ARDS’ low incidence and high case fatality, and based in earlier results, we expected that, even with a small sample size, Surfacen would have a positive effect on survival, and in no case would have a negative effect.
In the experimental group, treatment efficacy was assessed one hour and eight hours after each dose of Surfacen. In the control group, assessments were performed at the same intervals following initial assessment, which included arterial blood gas testing and mechanical ventilation settings.
Normality tests were conducted to confirm t-test requirements. When necessary, we used a Mann–Whitney–Wilcoxon rank-sum test, a nonparametric t-test substitute that has the advantage of not depending on an assumption of normal distribution. For quantitative variables, we calculated descriptive statistics (means and censored and uncensored medians, the latter with confidence intervals and standard deviations), overall and for each treatment group. Qualitative variables were summarized as absolute and relative frequencies.
The Fisher exact test was used to confirm the assumption of independence in 2 x 2 contingency tables. Confidence intervals were estimated for differences between proportions and odds ratios, using the normal approximation to the binomial distribution. The chi-square test for independence versus homogeneity was used with n x 2 contingency tables. The Mann–Whitney–Wilcoxon rank-sum test was used instead of chi square if cell sample sizes were small.
Number needed to treat was used to interpret the survival table for each treatment group. Log-rank analysis was used to compare survival curves.
Data processing was performed using SAS for Windows 9.1.3. Windows SPLUS 6.2 was used to generate graphics. Differences were considered statistically significant when p was <0.05.
Ethics The research protocol and supplemental documentation were designed following the specifications of the Guidelines for Good Clinical Practice in Cuba[26] and the Ethical Principles for Medical Research Involving Human Subjects (Declaration of Helsinki), amended by the 64th World Medical Association General Assembly, 2013.[27] Participating hospitals’ ethics committees approved the study protocol (reflected in Cuba’s Public Registry of Clinical Trials, RPCEC00000163).[28] Patient enrollment began once CECMED authorized the study.[29] Legal representatives (parents or guardians) provided written consent after being given oral and written information about the study in the presence of a witness.
RESULTS
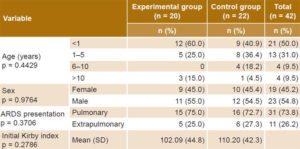
Table 1 displays experimental and control group demographics and initial values for clinical variables, demonstrating that the groups were substantially similar. Most patients were infants aged less than one year, and pulmonary forms of ARDS predominated. In some patients, more than one cause was reported at enrollment: pneumonia (30 patients; 14 in the experimental group and 16 in the control group), sepsis (29 patients; 15 experimental and 14 control), septic shock (22 patients; 11 in each group) and bronchial aspiration (one patient in the experimental group).
Table 1: Clinical and demographic characteristics of patients at enrollment
Efficacy Table 2 displays analysis of the main dependent outcome, based on 41 patients for whom information was available. Survival in the experimental group was 80% (16/20) and 38.1% (8/21) in the control group. For every 2.38 patients treated, one additional survivor was obtained in the experimental group. Uncensored median survival was greater in the experimental group than in the control group (34 days versus 12 days, p = 0.013 log-rank test). Figure 1 shows the survival curve for the experimental group, which remained above that of the control group through day 28 after enrollment. It should be noted that two patients (one in each group) died after the final date set in the protocol for assessing this outcome.
When we reviewed autopsy results to analyze cause of death (Table 2) and possible associations with the investigational drug, Surfacen, we confirmed that no death was caused by its use. All deaths were explained by patient clinical condition and underlying disease.
There were significant differences between the treatment groups concerning changes in gasometric and ventilatory outcomes, all favoring the experimental group (Table 3). There was no difference in Kirby index (p = 0.2786) between the two groups on initial clinical assessment, but differences were seen starting from first day that Surfacen was initiated. Kirby index surpassed 200 by the fourth dose (at 32 hours) in the experimental group and remained >200 thereafter (Figure 2).
Table 2: Patient vital status on day 28* and causes of death
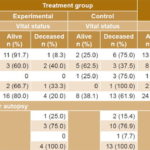
*p = 0.0074 (Fisher exact test) MOF: multiple organ failure
Radiographic findings in the two groups were similar on the first day following treatment initiation (p = 0.8072) (data unavailable for 2 patients who died in the first 24 hours). Progression of pulmonary infiltrates was seen in 59% of all patients (23/39); 33.3% (13/39) showed no change; and 7.7% (3/39) showed radiographic improvement (Table 4). Subsequent assessments found significantly better progress in the experimental than in the control group.
Hospital indicators showed no differences between the two groups. Mean mechanical ventilation time was 13.8 days in the experimental group and 14.1 in the control group (p = 0.2364). Mean PICU stay was 16.1 days in the experimental group and 15.2 in the control group (p = 0.3008).
The percentage of patients in the experimental group who responded to treatment (according to the Kirby index) was 75% (15/20) and 23.8% (5/21) in the control group. This difference was significant (p <0.001 Fisher exact test). The probability of response in the experimental group was 3.1 times (CI 95% 1.5–7.4) that of the control group.
DISCUSSION
Age distribution of patients enrolled in this study was similar to that of other studies,[6,30] in which the highest percentage of patients with ARDS fell in the group aged <5 years, especially the group aged <1 year. This may be linked to immaturity of the immune system at these ages, with resulting deficiencies in lactoferrin, lysozyme, defensins, collectins and immunoglobulin A, as well as mucociliary clearance abnormalities, which makes these patients more susceptible to serious infections.[1]
The predominance of pulmonary ARDS is consistent with similar studies that describe pneumonia and sepsis as the most common presentations.[1,7,19] Clinical trials with various exogenous surfactants[6,9,31] have shown that better response was achieved in patients with direct lung injury than in those with systemic disease. This can be explained by the fact that in systemic injury, the alveolar–capillary membrane allows plasma proteins and fatty acids to enter. Moreover, endothelial injury causes disorganized release of lytic enzymes, oxygen free radicals and nitrogenated species. These effects of endothelial injury inactivate endogenous surfactant and serve as a substrate for bacterial ischemia, reperfusion and translocation mechanisms during the course of ARDS.[31]
Achieving oxygenation stability during the first few days of ARDS is a challenge for clinical investigators, in our study reflected in the fact that most deaths occurred in the first few days after patient enrollment, regardless of randomization group.
Figure 1: Survival curve by treatment group
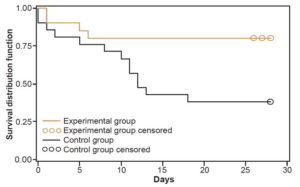
Table 3: Changes in gasometric and ventilatory indicators by treatment group
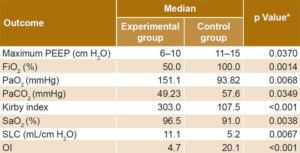
*Wilcoxon test FiO2: inspired oxygen fraction OI: oxygenation index PaCO2: arterial carbon dioxide tension PaO2: arterial oxygen tension PEEP: positive end-expiratory pressure SaO2: transcutaneous oxygen saturation SLC: static lung compliance
Figure 2: Kirby index* at time of treatment administration
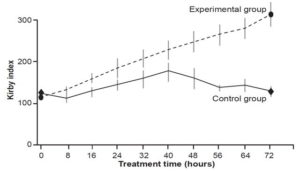
*arterial oxygen tension/inspired oxygen fraction
Our results suggest that Surfacen has a positive impact on ARDS survival. Reports from other studies of surfactants and ARDS survival are contradictory, with some indicating that surfactant treatment increases survival,[7,32] and others finding no effect.[19,33]
Table 4: Radiologic course by treatment group and response
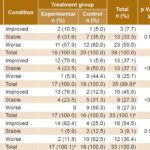
a On day 2, 2 patients died in each group. b Rounding error c On day 3, 2 patients died in the control group.
Overall case fatality in our study is consistent with reports from other countries that it is ≥50%, regardless of treatment strategy.[34,35] Exceptionally, Willson reports overall case fatality of approximately 25%.[7]
A recent paper reported no significant difference in case fatality for patients randomized to a group that received a combination of conventional therapy plus the surfactant Lucinactant, versus control patients who received only conventional treatment. Lucinactant is a synthetic surfactant, however,[36] unlike Surfacen.
Some authors posit that ARDS case fatality is due to hypoxemic injury, which causes irreversible deterioration of the respiratory cell unit, leading to the need for a respiratory support and mechanical ventilation to prolong life; in this conception, ARDS fits the category of multiple organ failure triggered by decreased tissue oxygen availability, leading to cell injury and ultimately, organ failure.[37–40]
Combining Surfacen with conventional treatment was effective because it increased arterial blood oxygenation in patients treated at the dose and frequency used. This is consistent with radiographic course, in which we noted considerable improvement in the number of collapsed areas of laminar or total atelectasis with no gas exchange,[10,41] as well as decreased inflammation in experimental group patients. Willson and Notter’s review of the topic states that lung aeration changes are clear in x-rays, and reflect improved oxygenation associated with administration of exogenous pulmonary surfactant.[32]
While earlier oxygenation assessments showed no significant differences between the treatment groups, differences in OI and SLC were seen after the fourth assessment, which is consistent with hemodynamic stabilization in the experimental group. The treatment model of repeated low doses also improved PaO2 and FiO2 values, a basic indicator of survival. Other authors have obtained similar results, finding evidence that exogenous surfactants have a positive effect on oxygenation.[5,6,31,42]
PaO2 and SaO2 value improvements resulted from reestablishment of alveolar oxygenation functions in the experimental group. These functions are associated with a significant decrease in FiO2, which help prevent oxygen toxicity and allow improved recovery compared to control patients treated with high oxygen levels for longer periods.[34,35]
PEEP has been used to improve arterial oxygenation for over two decades.[3,4] PEEP levels in patients in the experimental group were significantly lower than in the control group, from which we can infer that Surfacen achieves alveolar recruitment and opens collapsed alveolar populations, as well as keeping alveoli open longer, as evidenced by increased SLC and OI and decreased FiO2. Several authors agree that determining optimal PEEP is not simple, and advocate more for individualized treatment, with high PEEP when lung injury in the exudative phase allows recruitment, and low PEEP for very stiff lungs in the fibrotic stage, to prevent airspace collapse and alveolar shear stress phenomena.[1,43–45]
Improvements in Kirby index, OI and SLC are evidence of treatment effectiveness in increasing oxygenation.[1] By the second day of treatment, there were already significant differences between the study groups for these indicators, demonstrating that the investigational treatment was more effective than conventional treatment. Patients in the experimental group reached levels close to physiologic values, unlike in the control group, where there were indications of stabilization, but not improvement. The Kirby index and OI are the indicators most often used to evaluate effectiveness of exogenous surfactants, regardless of the age group in which they are administered, since this ratio changes due to lung dysfunction and several ventilatory variables.[32]
The significantly better radiographic progress in the investigational group was an unexpected result, given the slow rate of change in this outcome and the fact that a clinical trial of Surfacen in adult ARDS found no differences in radiographic course between groups.[25] Nevertheless, it is plausible. The function of pulmonary surfactant is to achieve alveolar interdependence (through the biophysical characteristics of the surfactant, which lowers surface tension in the collapsed area, causing simultaneous and equal expansion of all recruited alveoli), preventing some alveoli from inflating while others remain collapsed.[12,46].This is why the radiographic course for patients in the experimental group changed beginning on the second day of treatment, along with improvements in compliance and oxygenation index. Radiographic improvement is associated with recovery in the atelectasic areas, which causes radiopaque areas to become transparent, reflected in notable improvements in both clinical status and oxygenation gasometry.
The immunomodulatory,[21] antibacterial[22] and biophysical[12] properties of the surfactant used in this study contributed to improved lung function. This was due to its alveolar stabilizing effect, through reducing capillary–alveolar edema, and its demonstrated antibacterial effect on gram-positive and gram-negative microorganisms that cause deterioration of lung parenchyma.[22]
Hospital indicators (mechanical ventilation time and PICU stay) are outcomes assessed in many clinical trials with pulmonary surfactants. There is a wide variety of results, and as with other outcomes, they depend on dosage, treatment regimen, route of administration, type of surfactant, and ARDS etiology, among other factors.[32,35,47]
In his meta-analysis, Duffett found that pulmonary surfactant therapy was significantly associated with reduced case fatality and shorter mechanical ventilation time, although no differences were found in PICU stay.[48] Hong found a significant reduction in both mechanical ventilation time and PICU length of stay.[49] Our results are more in keeping with those of Thomas, who found no decrease in duration of mechanical ventilation.[36]
Our small sample size is a fundamental but unavoidable limitation, due to the high mortality of the disease. Even with this small sample, our results support the efficacy of Surfacen in this context, and CECMED has therefore approved the use of Surfacen in children with ARDS as a new therapeutic indication, after assessing all documentation generated in the clinical trial.[21]
CONCLUSIONS
Our results suggest that combining Surfacen with conventional therapy in the treatment regimen used improves oxygenation and increases survival in children with ARDS.
ACKNOWLEDGMENTS
The authors thank the researchers and PICU nursing staff who participated in this trial.









