INTRODUCTION
Gestational diabetes (GD) is the most common endocrine disorder affecting pregnant women and its prevalence is on the rise. Prevalence in Cuba is about 5.8%; global prevalence estimates range from 2% to 18% depending on the criteria applied.[1–3] According to the International Diabetes Federation, 382 million people worldwide had diabetes mellitus (DM) in 2013, and by 2035 that number will rise to 471 million.[4] Cases of diabetes that appear years after gestational diabetes will contribute to this increase.
GD’s rise, including in Cuba, responds to trends in certain risk factors in women of childbearing age, such as delayed pregnancy (aged ≥30 years) and overweight.[5] Other risk factors include: history of DM in a first degree family member, personal history of GD, a previous child with neonatal macrosomia, history of unexplained fetal death (especially after week 34 of gestation), pregnancy-induced hypertension, and polycystic ovary syndrome.[5,6,7] In Cuba, fasting plasma glucose (FPG), results are considered a risk factor if they are in the range of 4.4–5.5 mmol/L. Cuba’s National Comprehensive Diabetes and Pregnancy Program (PNAIGD) recommends an immediate oral glucose tolerance test (OGTT) for pregnant women with FPG results in the risk range.[6,7]
GD, which generally appears by week 24 of gestation, is asymptomatic for the mother but can affect the fetus. The most common and serious complication is fetal macrosomia, which is associated with traumatic or mechanical injury (shoulder dystocia, bone fracture, cephalohematoma, and brachial plexus or solid organ injury). GD can also result in metabolic disorders (hypoglycemia, hypocalcemia and hypomagnesemia), respiratory ailments (perinatal asphyxia and respiratory distress syndrome), cardiovascular disorders (diabetic cardiomyopathy and cardiopulmonary lability), hematological disorders (polycythemia and hyperbilirubinemia) and sepsis.[8]
Because GD is asymptomatic, clinical laboratory testing plays a crucial role in its screening and diagnosis. In Cuba, these activities are the responsibility of primary health care professionals (mainly family physicians), who thus need to be familiar with GD risk factors and screening for pregnant women at risk. The algorithm consists of an FPG test at the first prenatal visit, another at week 24 of pregnancy, and an OGTT between weeks 28 and 32, applying Cuban diagnostic criteria.[6,7] The purpose of this paper is to describe current international criteria for GD screening and diagnosis, present Cuban criteria and discuss the differences.
CONTROVERSIES ABOUT GESTATIONAL DIABETES
There is debate about several aspects of GD diagnosis, including: number of grams of glucose to administer, number of times to measure blood glucose in the OGTT, specific threshold values for diagnosis and number of measurements needed to determine abnormal results. There are also disagreements about screening approaches, whether screening should be selective or universal, and the specific screening test to use. Discrepancies even exist over how to label the condition of pregnancy-associated hyperglycemia.
Despite some disagreements, world experts do agree on some points, recognizing that GD:
- is a distinct diagnostic entity with specific risk factors;
- should be diagnosed and treated early (by about week 28 of pregnancy);
- can be treated with insulin;
- is associated with adverse gestational outcomes;
- can reoccur (in 70% of cases); and
- poses long-term cardiometabolic risk for both mother and child.[5,7–9]
Terminology GD emerged as a disease entity in 1979 at the First International Workshop Conference on Gestational Diabetes and in the early 1980s there was controversy over what term to use. As well as GD, it was variously called “carbohydrate intolerance in pregnancy” and “gestational diabetes mellitus.” The term GD had been used by Danish physician Jorgen Pedersen since 1967, but it was Norbert Freinkel and his collaborators in Chicago who promoted its use after the First International Workshop Conference.[10] GD was included with this name in the classification of DM by the National Diabetes Data Group (NDDG) in the United States in 1979.[11] It became the fourth subgroup in ADA’s classification of DM in 1997 (still in effect today).[12]
The Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study, led by the International Association of Diabetes and Pregnancy Study Groups (IADPSG), was completed in 2008. This seven-year prospective observational study was a multicenter (15 centers) and multiethnic (nine countries) program that recruited 23,316 women. Its purpose was to estimate risk for adverse perinatal outcomes depending on varying increases in maternal blood glucose values.[13] After the study’s results were published, it was suggested that “diabetes” is not a proper description of the condition of a strong, continuous and graded correlation between rising maternal glucose levels and increased neonatal weight, with its associated problems. For this reason, HAPO used the term “hyperglycemia in pregnancy,” reasoning that the hyperglycemia was less severe than in DM but still associated with increased risk for adverse gestational outcomes.[13]
Diagnosis O´Sullivan and Mahan proposed new criteria for assessing glucose intolerance in pregnancy in 1964 through application of an OGTT (Table 1),[14] later modified by Carpenter and Coustan in 1982,[15] based on risk of DM after a GD-complicated pregnancy. O´Sullivan also established the first screening test for GD (known as the O´Sullivan test), which in some places is still in use today. Its application does not require the patient to fast. The test consists of oral administration of 50 g of glucose diluted in water and measurement of blood glucose one hour later. Results are considered abnormal if the level is ≥130 mg/dL (7.2 mmol/L), with a sensitivity of up to 88%; or ≥140 mg/dL (7.8 mmol/L), with a sensitivity of approximately 85%.[16,17]
Table 1: Diagnostic criteria for gestational diabetes
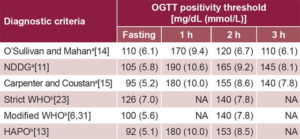
a≥2 elevated values are required to diagnose GD
bA single elevated value is sufficient to diagnose GD
GD: gestational diabetes
HAPO: Hyperglycemia and Adverse Pregnancy Outcomes
NA: not applicable
NDDG: National Diabetes Data Group
OGTT: oral glucose tolerance test
Proper application of the O´Sullivan and Mahan OGTT calls for measurement (using the Somogy-Nelson whole-blood reductive method) of initial fasting glucose and 1, 2 and 3 hours after administration of 100 g of glucose.[13] Use of O´Sullivan’s screening method for pregnant women, followed by the diagnostic test (O´Sullivan and Mahan’s OGTT) for patients whose screening results showed altered levels, is known as the two-step method.[18] In 1979, in light of a new technique of measuring glucose in blood plasma, the NDDG adapted the original O´Sullivan and Mahan threshold values (from whole blood analysis) (Table 1) to the new laboratory method, which involved an increase in threshold values.[10,11]
Subsequent proposals by Carpenter and Coustan—approved in 1997 at the Fourth International Workshop Conference on Gestational Diabetes[19] and in 2000 by ADA[20] and American College of Obstetricians and Gynecologists (ACOG) in 2001[21]—introduced changes made possible by advances in clinical laboratory procedures. Glucose could be measured in blood plasma by the enzymatic glucose oxidase method, leading to lower diagnostic threshold values in comparison with NDDG thresholds (Table 1), and consequently, a spike in estimated GD prevalence. For this reason, some countries, such as Spain, never adopted Carpenter and Coustan’s diagnostic criteria and instead opted to continue to apply the NDDG criteria.[22] Blood glucose threshold levels in the 3-hour OGTT to diagnose GD have varied at each of these 3 points in history: 1964 (O´Sullivan and Mahan),[14] 1979 (NDDG)[11] and 1997 (Carpenter and Coustan).[15]
Since late 2010, IADPSG and ADA have recommended a 75-g, 2-hour OGTT to diagnose GD using threshold values from the HAPO study, based on increased risk of adverse perinatal outcomes with rising maternal blood glucose. Their current recommendations differ from previous ones in dose (75 g instead of 100 g) and in that blood glucose is measured 3 times instead of 4 (fasting, and at 1 and 2 hours), with a single elevated level (rather than ≥2) required to diagnose GD.[12,19] These criteria were recently approved by WHO,[23] the Endocrine Society,[24] International Diabetes Federation[25] and International Federation of Gynecology and Obstetrics (Table 1).[26]
Under these new criteria, estimated GD prevalence is expected to rise, which could lead to increased health care costs.[13] Several countries—including India (Diabetes and Pregnancy Study Group),[27] and the UK prior to 2015 (National Institute for Health and Clinical Excellence, NICE)[28]—recommend strict application of WHO’s 1999 diagnostic criteria.[23] NICE, however, currently recommends modified WHO criteria (Table 1).[28]
Screening Another source of controversy has been whether screening should be universal or selective (for at-risk pregnancies only) or even whether screening should be performed at all. The Second International Workshop Conference on Gestational Diabetes approved universal screening.[10] At the fourth workshop conference, however, it was proposed that low-risk pregnancies be excluded (selective screening). Low risk was defined as pregnancies that meet the following conditions: aged <25 years, appropriate prepregnancy weight (body mass index <25), belonging to ethnicity or group with low DM prevalence, no history of DM in first-degree family members, and no history of glucose intolerance or adverse obstetrical outcomes. At that time, ADA approved the proposal but ACOG did not, based on the argument that 3% to 10% of GD cases would be missed.[17–19,21] At present most countries do not adhere to universal screening but rather perform mass case-finding, which consists of conducting diagnostic tests (without initial screening) on all pregnant women as recommended by IADPSG[13] and ADA,[20] although its cost-effectiveness remains to be proven.[9,16] Spain, however, continues to follow ACOG recommendations for universal two-step screening.[22]
One concern about use of FPG as a screening test is that it is less sensitive (around 82%) than the O’Sullivan test (88%–99% or 70%–89% using cutoffs of 130 mg/dL and 140 mg/dL, respectively).[16,17] It has not been determined whether the IADPSG and ADA recommendations for actively searching for GD in all pregnant women is beneficial.[9] The test has moderate sensitivity (82%) that decreases as pregnancy advances, since the fetus consumes large quantities of maternal glucose, accounting for pregnant women’s generally low FPG.[7,8,29,30] OGTT administration between weeks 24 and 28 (as currently recommended by IADPSG, ADA and the Latin American Diabetes Association [ALAD]) enables early dagnosis of GD, and as a result, early therapeutic intervention, which theoretically should benefit both mother and child. Treatment is considered early if it begins before week 30 of gestation.[7,9,21,31,32]
In the USA, GD has traditionally been diagnosed through use of a two-step method: first a screening test (the highly sensitive O’Sullivan test), followed by a diagnostic OGTT for women with abnormal screening results.[10,16,18,21] This method is currently recommended by ACOG,[33] but not by IADPSG or ADA, which recommend omitting the screening step and going directly to the diagnostic test between weeks 24 and 28 for all pregnant women.[13,20] Others object that, because two-hour postprandial blood glucose is highly variable and only one abnormal value is required to make the diagnosis, this method results in a higher number of false positives than does the two-step method.[9]
Cuban policy Diagnosis Modified WHO criteria are used in Cuba to diagnose GD (Table 1): ≥2 FPG tests ≥5.6 mmol/L or a single 75-g 2-hour OGTT ≥7.8 mmol/L.[6,7 ] This diagnostic method for GD is identical to the recommendations of the 2007 Consensus Statement on Diabetes and Pregnancy in Latin America issued by the XIII ALAD, which is still in effect today.[31] It is similar to the current ADA recommendation, derived from the HAPO study, but uses different threshold values (Table 1) and does not include one-hour blood glucose levels (Table 2).[13,20]
Although IADPSG and ADA consider a glucose metabolism disorder diagnosed in the first trimester of pregnancy as preexisting DM,[13,20] in Cuba, any hyperglycemic condition appearing during pregnancy is considered GD, independent of the trimester in which it was detected.[6,7] Since the end of 2010, with the first Cuban consensus on prediabetes, diagnostic criteria for GD have been harmonized with Cuban diagnostic criteria for different categories of glucose intolerance and DM in the general population. As a result, prediabetes and diabetes appearing during pregnancy are considered to be GD (Table 2).[6,7,29] This extremely comprehensive criterion means that the following cases are considered to be GD: (a) prepregnancy DM diagnosed during pregnancy, including types 1, 2 or other; and (b) typical GD, which is an asymptomatic disease generally appearing after the 24th week of pregnancy associated with hormonal changes occurring during pregnancy.[7,8,18]
Table 2: Cuban and international approaches to GD detection

aScreening followed by OGTT if FPG is 4.4–5.5 mmol/L
bOnly women at risk for GD; in low-risk pregnancies if FPG is 4.4–5.5 mmol/L
ACOG: American College of Obstetricians and Gynecologists
ADA: American Diabetes Association
ALAD: Latin American Diabetes Association
DIPSI: Diabetes in Pregnancy Study Group India
FPG: fasting plasma glucose
FIGO: International Federation of Gynecology and Obstetrics
GD: Gestational diabetes
HAPO: Hyperglycemia and Adverse Pregnancy Outcomes
IADPSG: International Association of Diabetes and Pregnancy Study Groups
IDF: International Diabetes Federation
NDDG: National Diabetes Data Group
NICE: National Institute for Heath and Clinical Excellence, UK
OGTT: oral glucose tolerance test
Screening Universal screening for GD with FPG has been in place in Cuba since the 1990s. PNAIGD recommends that all pregnant women have an FPG test at their first prenatal visit (enrollment) and another around week 28 (reassessment consultation); measurements ≥5.6 mmol/L are considered GD; values of 4.4–5.5 mmol/L are considered a GD risk factor and screening tests in this range are immediately followed by the diagnostic test, a 75-g 2-hour OGTT; measurements ≥7.8 mmol/L are considered elevated (modified WHO diagnostic criteria, Table 1). Most experts recommend that screening and diagnosis be conducted between gestation weeks 24 and 28, when the hyperglycemic effect of gestation has already developed).[17,20,24–26,31,32] In at-risk pregnancies in Cuba, FPG is ordered at the first prenatal visit and repeated at week 24; if GD is not detected by weeks 28–32, a 75-g 2-hour OGTT is ordered, not preceded by another screening test, as in the two-step method.[6,7,29,30]
The majority of pregnant women in Cuba are diagnosed with GD between weeks 24 and 27 of gestation and have previously shown signs of impaired FPG in a screening test (4.4–5.5 mmol/L). The rest are diagnosed before week 24 or between weeks 28 and 32, whether or not preceded by a positive screening test.[34] Application of a GD screening test without high sensitivity at week 24 would miss borderline cases, which would inhibit early diagnosis and treatment and adversely affect pregnancy outcomes.
Nevertheless, no Cuban studies have shown conclusively that women diagnosed with GD between weeks 24 and 28 have better maternal and perinatal outcomes than those diagnosed later. Although a small study conducted in 2016 on 302 women with GD found that diagnosis before week 28 was associated with earlier therapeutic intervention, there was no association with gestational outcomes, nor were significant differences found between groups in delivery timing (term or preterm), whether or not delivery was dystocic, one-minute Apgar score or fetal macrosomia.[34]
FINAL CONSIDERATIONS
International consensus has not yet been reached on several issues related to GD screening and diagnosis, in which clinical laboratory testing plays a critical role. New regional and international meetings are needed to reach agreement on these differences, based on different countries’ experiences. Pregnancy outcomes for Cuban women with DM (including GD) are satisfactory, with national rates similar to those elsewhere in the Americas for preeclampsia (5%), preterm delivery (12%), neonatal macrosomia (7.5%), congenital anomalies (4.3%) and perinatal death (4.8%).[35] Thus, while Cuban criteria differ in some respects from international approaches, there is no evidence that a drastic overhaul is needed.





