INTRODUCTION
Over the past two decades, chronic kidney disease (CKD) has emerged as a health problem of epidemic proportions in a number of rural areas.[1] The epidemic affects primarily young men living in farming communities in Central America,[2,3] specifically El Salvador,[4–7] Nicaragua[8–10] and Costa Rica,[11] as well as in other countries: Sri Lanka,[12–14] Egypt[15,16] and India.[17,18] This CKD, in most cases, is not due to diabetes mellitus, hypertension, primary glomerulopathy or obstructive uropathy, the main causes of CKD in adults, nor have any other causes been established with certainty. This has led many to call it chronic kidney disease of nontraditional causes (CKDnt).
However, several hypotheses have been generated about the causes of CKDnt. The two considered most plausible, not necessarily mutually exclusive,[19] are outlined below.
The first hypothesis holds that CKDnt is caused by occupational exposure to agrochemicals used indiscriminately and without protection during farm work, and from environmental exposure to pollutants in soil, water, air and food. This exposure is potentiated by intense labor at high temperatures coupled with inadequate hydration (leading to toxin concentration in the renal medulla), and is associated with social determinants, notably poverty.[6,20]
The second hypothesis does not include agrochemicals as a causal factor but attributes the illness to strenuous working conditions, high temperatures and consequent dehydration, without adequate replacement of fluids and electrolytes, which could cause repeated acute kidney injury, in turn eventually leading to chronic kidney damage.[21,22]
Studies of renal biopsies from adults in Sri Lanka,[23] Costa Rica[11] and El Salvador[24,25] suggest that kidney damage is a chronic tubulointerstitial nephritis with accompanying glomerular and vascular changes.
Most epidemiological studies of this health problem in Central American countries have focused their attention on male sugarcane farmers. In El Salvador, several studies have also reported the disease in farmers of crops other than sugarcane, in nonfarming men and in both farming and nonfarming women.[20,24,26,27] In a case–control study in Nicaragua of adolescents without an occupational history in agriculture, increased average levels of interleukin 18 (a tubular damage marker) were more frequent in female than in male adolescents.[28] Presence of this tubular damage marker in adolescents resembles the findings of clinical and pathological studies in adults in Central America.[20,24,25]
Although CKDnt mainly affects adults, a recent study in Nicaragua established the presence of markers of renal tubular damage in adolescents aged 12–17 years in schools located in regions with high CKDnt prevalence. Adolescents were excluded if they had worked at manual labor for a month or more, paid or unpaid. The authors concluded that finding elevated urinary levels of neutrophil gelatinase associated lipocalin (NGAL) and N-acetyl-beta-D-glucosaminidase (NAG) proteins suggested the possibility of kidney injury prior to occupational exposure.[29]
In farming communities in the Bajo Lempa region of El Salvador, a CKD prevalence of 17.9% (25.7% in men and 11% in women) has been documented in adults (aged ≥18 years). These rates are far higher than those found in other CKD studies among adults in various countries,[5] where typical rates range from 10%–13%.[30] In over 50% of Salvadoran participants, CKD was not due to any of the known or traditional causes. Prevalence of chronic renal failure (CRF) is also high in this region: 9.8% (18.9% in men and 4.1% in women).[6]
Globally, epidemiological information on renal damage in the pediatric population is more limited. Unlike adults, where the greatest proportion of CKD is due to diabetes and hypertension,[30] in childhood, it is mainly attributed to congenital disorders in developed countries and to infections or other acquired diseases in developing countries. Estimates of chronic kidney disease prevalence in pediatric populations range from 21 to 108 per million.[31]
The kidneys are susceptible to toxic damage due to, among other factors, increased renal perfusion. The high metabolic activity of the proximal tubular epithelium makes it particularly susceptible to toxic injury, though other parts of the nephron can also be affected.[32] Moreover, high exposure to numerous known nephrotoxic agents would have a direct, acute effect on the pediatric population. A growing body of literature supports the hypothesis that low-level exposure to several nephrotoxins can also increase risk of CKD or accelerate its progression.[32] Prenatal exposure to nephrotoxins during renal system development can reduce nephron mass, manifested as changes in renal structure and function.[33] A recent study in China detected an average of 15.3 pesticides per sample in cord blood from 336 neonates.[34]
The NefroSalva Pediatric Study’s objective was to determine the prevalence and distribution of CKD and of markers of kidney damage in the child and adolescent population of Salvadoran farming communities, with a view to supplementing insights into the epidemic from studies in adults.
METHODS
The study was conducted by the Renal Health Research Unit in the National Health Institute of El Salvador’s Ministry of Health. The research team included physicians, nurses, clinical laboratory technicians, epidemiologists, community health workers, staff from the Health Solidarity Fund of El Salvador, Salvadoran nephrologists and students from the University of El Salvador Medical School and Cuba’s Latin American Medical School, with active participation by community health committees in the areas studied. Experts from the Nephrology Institute and National School of Public Health of Cuba’s Ministry of Public Health served as PAHO advisors in the framework of intercountry technical cooperation.
Study type A descriptive study was conducted from 2009 to 2011, through active screening for cases of CKD and urinary markers of kidney damage in the population aged <18 years in farming communities in 3 regions of El Salvador: Bajo Lempa (Usulután Department), Guayapa Abajo (Ahuachapán Department) and Las Brisas (San Miguel Department).
Setting Bajo Lempa is a farming region located along the banks of the Lempa River near the southeastern coast of El Salvador.[35] Guayapa Abajo is also a farming region (known for its sugar cane), on the southwestern coast. Las Brisas is a periurban farming region in eastern El Salvador, close to the city of San Miguel (in the department of the same name). There is high prevalence of CKD in all three regions.[6]
Three general conditions characterize the regions in the study: high levels of poverty, unhealthy working conditions,[20,35] and an environment contaminated with pesticide residue and, in the case of Bajo Lempa and Las Brisas, also heavy metals.[36–39] Furthermore, working conditions for farm laborers are characterized by indiscriminate use of agrochemicals (combining several at once, some banned, used without protection with consequent environmental pollution)[36] and heavy physical labor (causing profuse perspiration) for many hours in high temperatures and without adequate hydration.[20]
The study had two phases:
- A CKD screening phase, involving one-time testing for urinary markers of kidney damage and estimation of renal function.
- A CKD confirmation phase, three months following the first phase, to validate positive findings for the above parameters.
Study population A door-to-door census of 11 communities belonging to the 3 study regions identified 5018 individuals of all ages (1306 families). Of the 2163 children and adolescents, 2115 (97.8% of the population enumerated) aged <18 years were studied (1058 male and 1057 female). Urine samples for CKD marker studies were available in the confirmation phase from 1755 individuals, blood samples for creatinine determinations from 1960, and both samples in 1623.
Study variables are described in Table 1.
Recording and coding Each participant was assigned a database code for subsequent clinical management. General information was obtained via a questionnaire and physical parameters were measured to determine glomerular filtration rate (GFR).
Laboratory tests A first morning urine sample was tested using Multistix 10 SG (Bayer, USA) test strips, to rule out urinary infection and hematuria. If neither was detected, Microalbumin 2 (Bayer, USA) reagent strips were used to determine the albumin:creatinine ratio (to assess albuminuria). Test strips were read using a Clinitek (Bayer, USA) analyzer.
A 5-mL fasting venous blood sample was drawn from participants to measure creatinine by the enzymatic method. Samples were processed in a laboratory installed in each region and equipped with a Cobas C111 spectrophotometer (Roche, Germany), with its corresponding reagents and quality controls. Laboratory tests were performed per manufacturers’ specifications using appropriate controls. Measurements and analyses were done by trained and certified personnel.
Table 1: Variables

ACR: albumin/creatinine ratio CKD: chronic kidney disease GFR: glomerular fi ltration rate KDIGO: Kidney Disease Improving Global Outcomes
Data management and analysis Data were stored in a Microsoft Excel database processed with SPSS version 23.0 for Windows. General prevalence of urinary markers of kidney damage was calculated as were specific CKD prevalence rates by sex, age group and region. Hypothesis testing was done to compare means to respective pediatric reference values. Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines were used as normal reference values.[40] A 0.05 threshold was used to assess whether differences were statistically significant. GFR was calculated using the Schwartz formula[41] and the average was plotted by age group.
Ethics Written informed consent was obtained from all parents and participants respectively, who agreed to publication of the results under conditions of confidentiality. The study was approved by the Salvadoran Ministry of Health’s Executive Council. All participants received clinical followup by public health services.
RESULTS
Prevalence of urinary markers of kidney damage was 4% (95% CI 3%–5.8%) of the population studied; 4.3% in girls and 3.8% in boys. Table 2 displays detailed data on marker prevalence. Microalbuminuria was detected in both sexes and all regions, with prevalences of 2.6%–3.8% in boys and 3.3%–3.8% in girls. Macroalbuminuria was only detected in girls in Las Brisas (2.3%). The highest prevalence of hematuria was in boys in Las Brisas (1.6%). It was not detected in Bajo Lempa boys or in Guayapa Abajo girls. Testing with urine test strips found no proteinuria or evidence of urinary tract infection.
In comparison with normal reference values,[40] glomerular hyperfiltration was observed in all age groups and both sexes (Table 3). Consequently, an analysis was done comparing GFR with kidney damage markers, the latter found in 80% of children and adolescents with GFR ≥140 mL/min. Figure 1 shows average GFR by sex and age group.
Overall estimated CKD point prevalence was 3.9%. Prevalence in the group aged 2–5 years, 5.1%, was greater than in the other groups, but all CIs overlapped. The same happened with the regions. Similarly, prevalence in girls was greater than in boys (4.1% versus 3.6%), but with overlapping CIs. Las Brisas had the highest estimated CKD point prevalence, 4.3%, but its CI overlapped with those of the other two regions (Table 4). Two cases of CRF were found, a boy aged 13 years in stage 3a and a girl aged 10 years in stage 5, both from the Guayapa Abajo region.
DISCUSSION
This is the first population-based investigation of CKD prevalence, kidney damage markers and average GFR values in minors in El Salvador. It provides clear evidence that kidney damage often begins in childhood. The results are similar to those from screening in Guatemala in the population aged 6–18 years in 2014, which found a 5% prevalence of urinary markers of persistent kidney damage.[43] In the city of Jalisco (Mexico), screening in 2006–2007 found a 5% prevalence of persistent proteinuria and a 3.7% prevalence of GFR <80 mL/min in persons aged <18 years.[44]
In weighing the two causal hypotheses described above, it is worth noting that some population groups do not engage directly in farm work, such as preadolescent children as well as women and men who are not farmers. Thus, repeated kidney injury secondary to dehydration is not enough to explain CKD in these population groups. Logically, male and female farmers may suffer greater dehydration, and also exposure to toxic substances. However, there may also be environmental contamination that affects the population as a whole, associated with economic and social disadvantages that make these populations more vulnerable.
Reasonably, there may be different levels of exposure to toxins: the most damaging, high-level exposure would be repeated over time, consisting of multiple acute exposures that would ultimately produce a chronic ailment. This would primarily affect farmers, due to chronic circulation in blood of toxins that are eliminated by the kidneys, causing these toxins to concentrate in the renal medulla under the effects of long work hours in high temperatures with intense physical activity. Deficient hydration would potentiate the effects of such toxins. Furthermore, the general population might suffer lower-level chronic exposure, which could affect children, to a greater or lesser extent depending on their genetic susceptibility and other factors.
Table 2: Prevalence of kidney damage markers, by sex and region

Table 3: GFR compared to normal reference values (KDOQI) by region, age and sex
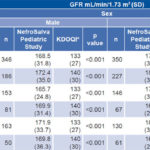
*reference values[40] GFR: glomerular fi ltration rate
With regard to the effects of pesticides on child and adolescent health, low-level chronic cumulative exposure may be associated with subclinical health effects with delayed consequences that do not appear until weeks, months or years later. This exposure may begin prenatally or in infancy,[45] consistent with our finding of hyperfiltration even in the very youngest group, aged 2–5 years.
In rural areas, proximity to farmlands where there is high pesticide use (including periodic applications near schools, homes and playgrounds) is conducive to children and adolescents coming into contact with pesticides in water, air, soil and food. In addition, domestic exposure occurs from pesticide storage in the home. Moreover, the farmers who apply pesticides without protection have their clothes drenched with them, providing a source of exposure to the family, especially women, who are exposed when washing contaminated clothing.[36,45] Other factors add to this cascade of circumstances, such as social determinants that make these communities more vulnerable to possible prior kidney damage: low birthweight, infectious diseases such as malaria and arboviruses, malnutrition and others. The data presented and environmental considerations persuade us of the importance of a possible multicausal etiopathogenesis centered on nephrotoxicity from agricultural toxins.
CKD prevalence in both sexes greater than reported internationally is consistent with observations in adults in the same regions. In Las Brisas, where the greatest prevalence of CKD has been found both in adults[6] and in children and adolescents, there is a storage warehouse for toxaphene, known for its severe effects on the kidney, liver and nervous system (banned by USEPA in 1990).[46] The abandoned depot was dismantled by Las Brisas residents. According to El Salvador’s Ministry of Environment and Natural Resources, in 2009, toxaphene residue was found in nine of ten wells tested.[47]
A recent study in three regions of Nicaragua demonstrated the presence of biomarkers of tubular renal damage, particularly NGAL and NAG, in regions with high mortality from CKDnt.[29] These findings correspond to the clinical and histopathological characteristics of the chronic tubulointerstitial nephritis described in adults studied in these Salvadoran farming communities.[20,24]
Glomerular hyperfiltration found in both sexes and both age groups in all the communities studied may reflect adaptation of renal function when a given toxin, of whatever sort, functionally destroys a large number of nephrons. However, it may also result from low birth weight’s effect on total nephron mass, producing congenital oligonephropathy;[48] i.e., decreased individual nephron endowment at birth, which increases susceptibility to CKD in adults.[49–51]
Figure 1: Mean GFR by age group and sex
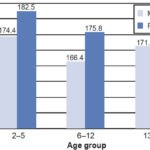
GFR: glomerular fi ltration rate
An important finding was that GFR began to decrease before age 13 years. The Nefrolempa study in adults found a monotonic decline in GFR throughout adult life, with worse renal function in men than in women, in all age groups.[5] Notably, in our study the age group 13–17 years was the only one in which girls had worse renal function than boys. This could be a cohort effect, but more extensive research is needed to be able to explain the pattern and this exception.
Table 4:Prevalence of chronic kidney disease by stage, age group, sex and region
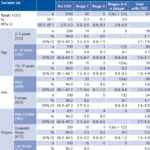
No similar data are available from other farming communities with high prevalence of CKDnt to enable comparison of the magnitude and distribution of CKD in pediatric ages. We were unable to find any published CKD prevalence study in children, although some studies exist of CRF and end-stage renal disease prevalence. A study in Italy found a mean incidence of CRF (defined as creatinine clearance <75 mL/min/1.73 m2 of body surface area) of 12.1 cases per year per million of the age-related population, with a point prevalence of 74.7 cases per year per million individuals aged <20 years.[52] It should be borne in mind that this study used an earlier, less restrictive definition of CRF.[53] In Sweden, a study in children aged 6 months to 16 years found CRF (with GFR <30 mL/min/1.73 m2 of body surface area) incidence and prevalence of 7.7 and 21, respectively, per million children.[54] The United States Renal Data System reports that among 31 countries, incidence of end-stage renal disease in children aged <20 years was the highest in Qatar (33.2 cases per million) while the highest prevalence was in the Basque Country (Spain) with 108 cases per million population.[31] The CKF prevalence we found (0.1%) is about 10 times the highest prevalence found internationally.[31] It is striking how debut and elevated prevalence of CKD at early ages mirrors prevalence in adulthood in these communities.
Among the limitations of this study, it should be noted that although the Schwartz formula[41] we used to estimate GFR in the pediatric population is the most widely used in epidemiological studies, it was designed for the US population. It should be validated in the Salvadoran population for greater reliability, or a new formula developed to estimate kidney function in Salvadoran children. Also, there is an apparent contradiction between the finding of macroalbuminuria in a small percentage of children and failure to detect proteinuria with the reagent strips, which can be explained by the relative insensitivity of the strip used for proteinuria.[55] Despite these limitations, the study is useful for estimating the magnitude of the problem and for generating research hypotheses.
The information obtained has been useful for planning programs to address the health care needs of the affected population. It was the basis for the prevention component in primary care launched by the Ministry of Health reform of 2009. Comprehensive care has been instituted in the regions studied. In Bajo Lempa, a community health team has been trained to provide prevention and treatment services. Methodological and practical lessons from this study have been extended to other areas and have facilitated new health screenings and interventions in other rural Salvadoran communities.
More in-depth studies are needed to establish causes of the disease and of the epidemic. These studies must consider environmental contamination by nephrotoxins as part of the explanation. For now, there is sufficient evidence of occurrence of CKD in childhood to justify clinical interventions in its early diagnosis, as well as timely treatment and rehabilitation. These actions can only be effective with an intersectoral approach that also addresses social determinants, especially those related to environmental control, best farming practices, labor conditions/occupational health and appropriate pesticide use.
CONCLUSIONS
Our results are consistent with the hypothesis that exposure to environmental toxins contributes to CKDnt in these Salvadoran communities, including children, drawing attention to the need for primary prevention starting at an early age.








