In 1981, Cuba’s biotechnology sector was born when Cuban scientists, trained by Finnish colleagues, successfully isolated large quantities of human leukocyte interferon alpha. Within a decade and following a $1-billion dollar investment by the Cuban government, 52 scientific research institutions were established in and around Havana to develop vaccines, therapies and other medical applications to meet demands of the national health system.
The strategy—based on a “closed loop” approach[1] whereby research, development, manufacturing, clinical trials, marketing, and postmarketing surveillance are coordinated by a single research institute in cooperation with others—has the first priority of forging effective solutions to Cuba’s most pressing health problems. Its second priority was, and is, to generate profits from sales abroad, which are largely plowed into further R&D and infrastructure, to make the industry independent of government financing over time.
Success came early and often: Initial achievements by Cuba’s biotech industry included the world’s first meningococcal B vaccine; PPG, a natural cholesterol-lowering drug derived from sugar cane; and a Haemophilus influenzae type B (Hib) vaccine created from synthetic antigens—a global first. Each of these innovations earned World Intellectual Property Organization (WIPO) Gold Medals: MENGOC-BC in 1989, PPG in 1996, and Quimi-Hib in 2005.[2] In the 1990s, after these and other products were introduced throughout the health system, it became clear Cuban biotech could positively impact population health not only at home, but abroad as well. Achieving this required an efficient, reliable clinical trial infrastructure in accordance with international protocols and best practices.
GOOD CLINICAL PRACTICE (GCP)
Good clinical practice is an international ethical and scientifi c quality standard for designing, conducting, recording and reporting trials that involve the participation of human subjects. Compliance with this standard provides public assurance that the rights, safety, and well-being of trial subjects are protected, consistent with the principles that have their origin in the Declaration of Helsinki, and that the clinical trial data are credible.
Good Clinical Practice: Consolidated Guidance, International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH)
CUBAN CLINICAL TRIALS: A BRIEF HISTORY
As Cuba’s biotech sector continued making scientific breakthroughs during the 1980s and into the early 1990s, research institutes developing new treatments worked directly with hospitals to conduct trials. Oversight of trials and introduction of new, approved medical applications into the health system were organized by the developing institution, in coordination with the country’s regulatory agency—the Center for Quality Control of Medicines, Equipment and Medical Devices (CECMED). Although there were some success stories, on the whole, this approach presented problems related to clinical trial design and implementation, combined with long lag times between scientific discovery and making new treatments available to patients. Moreover, on the global front, clinical trials were evolving quickly throughout this period, with an emphasis on trial efficiency and quality, combined with standardization of protocols across borders.
Internationally, clinical trial design, organization, and implementation were increasingly being handed over to commercial entities known as Contract Research Organizations (CROs), employed by drug developers to bring effective, innovative medicines to market as quickly as possible, while paying due attention to safety. Cuban scientists knew their clinical trial approach needed an overhaul and saw an opportunity to learn from these new enterprises.Implementing best practices according to international norms would enable the nation’s biotech sector to maximize its impact on population health, while positioning its products to compete overseas. In 1991, Cuba’s Clinical Trials Coordinating Center (CENCEC) was founded to design, coordinate and implement clinical trials to evaluate new drugs, vaccines, reagents and medical devices using “full ethical, scientific, and methodological rigor in compliance with international standards.”[3] While some research institutes (e.g., Finlay Institute) continue to design, coordinate, implement and monitor clinical trials of their products, including ethical approval, they all must adhere to the same Good Clinical Practice (GCP) that guides CENCEC standards. Evaluation in these cases is provided by the institution’s monitoring program, followed by a separate assessment by CENCEC’s Quality Control Division, upon request.
CENCEC was modeled after CROs, which conduct cost-effective, quality clinical trials quickly—elements important to Cuban scientists who, in their earlier experiences, saw many promising products in their biotech portfolio wither in the lab due to the high costs of conducting trials and insufficient scientific rigor in the design and evaluation stages. Nevertheless, Cuba’s universal health system, combined with the closed loop approach to scientific research, provides CENCEC advantages over traditional CROs: It is integrated and national in scope, couples scientific services with academic and health research, incorporates continuous specialist training, and prioritizes the country’s most pressing health problems as established by the Ministry of Public Health (MINSAP).
The gold standards guiding the founding of CENCEC and restructuring of Cuba’s clinical trial strategy included designing controlled, randomized and concurrent multicenter clinical trials applying a single protocol; accreditation of approved clinical trial sites; specialist training; quality assurance controls; rigorous data collection and analysis; and strict ethical conventions designed to protect trial participants and provide transparency. To achieve this, the following steps were taken, the majority of them beginning in 1992:
- The national regulatory framework was overhauled to include the independent regulatory agency (CECMED, founded in 1989) to assess preclinical data and recommend whether to proceed with developing a clinical trial protocol for the product in question.
- The National Clinical Trials Coordinating Network was established, based at medical universities (linked in turn to teaching hospitals) throughout the country, to coordinate participant recruitment, implement multicenter trials and conduct follow-up evaluations.
- The National Clinical Trials Site Network was set up to promote multicenter trials in order to satisfy product developers’ demand for participants, speed recruitment, and coordinate extension of trials and products to more sites throughout the health system.
- GCPs were implemented throughout the clinical trials program, including ethical considerations relevant to trials in humans; data collection, management and analysis; and post-trial evaluation.
- CENCEC’s Quality Control Division was created to provide independent evaluation of all stages of trials, from protocol design and writing to trial preparation, data collection and analysis, and preparation of the final report.
- A Clinical Trial Monitoring Program was developed, reporting to the Quality Control Division on the reliability and quality of information generated by each clinical trial.
- An Academic Development Strategy was designed and implemented, providing national specialist training at medical schools across the island, to train health professionals and scientists participating in clinical trials in internationally uniform protocols and keep them abreast of emerging trends in contractual clinical trial research.
- A certification program for clinical sites, under the auspices of CECMED, was developed, to ensure each site applied GCPs in their work.
- Some 120 Independent Ethics Committees for Scientific Research (CEI), were created across the country by the Ethical Evaluation Program, in accordance with WHO guidelines for clinical trial ethical oversight.
- A Medical Materials Assurance program was established to guarantee the resources (and their proper storage and distribution) required for design, conduct and evaluation of clinical trials.
- The Cuban Public Registry of Clinical Trials went live in 2007; in 2011 it became the first WHO-accredited primary registry in the region.
Before any clinical trial is launched, the sponsoring institution must submit to CENCEC their Strategy for Clinical Evaluation and Clinical Program for the product it wishes to test. This document must include: trial objectives, the product’s relevancy (i.e.: does it address one of the ten leading causes of mortality or morbidity in the country), feasibility, study type, timeline, cost and evaluation process. This document must also outline the plan for securing the necessary licensing so the product can be introduced into the national health system, as well as plans for insertion into the international market. Before receiving the green light, a selection of possible participants must be assured. Other considerastions include incidence of the condition or illness the trial targets and state of infrastructure and resources of potential trial sites, as well as professional capacity at these sites to recruit, evaluate and select participants. CIEs slated to implement the trials are actively involved in all facets pertaining to the scientific ethics of the trial in question, including obtaining informed consent; participants must submit written informed consent before trials can begin. Only clinical trials registered in the Cuban Public Registry of Clinical Trials can proceed—a requisite instituted after the Registry received certification as a WHO primary registry.
Timeline of Clinical Trials in Cuba

EARLY RESULTS
Once the infrastructure and regulatory framework were in place, Cuban research centers set out to test some of their most promising therapeutic and diagnostic products according to GCPs. Given that heart disease is a leading cause of death in Cuba[4] and that the aim of scientific research and development on the island is to improve population health, it’s not surprising that the first large-scale, multicenter randomized trial was conducted to test the safety and efficacy of recombinant streptokinase, a treatment for acute myocardial infarction derived from human DNA. Developed by the Center for Genetic Engineering and Biotechnology (CIGB), Heberkinasa underwent extensive clinical trials in Cuba between 1996 and 1999—at that time, the largest multicenter trial ever undertaken on the island, involving thousands of participants in 52 hospitals across the country. Coordination was handled by the National Clinical Trials Coordinating Network. This trial was especially challenging since to be effective, recombinant streptokinase must be administered shortly after a cardiac event. Results of the trial, as monitored and evaluated by the Quality Control Division and the Clinical Trial Monitoring Program, revealed a decrease in intrahospital mortality of over 10%. Heberkinasa has since been introduced at the primary care level in Cuba and is registered and sold in over 25 countries.[5]
Multiple sclerosis; Hepatitis C; schizophrenia; burns; deep vein thrombosis; lung, neck, head and breast cancers; chronic myeloid leukemia; respiratory papillomatosis; Infant Respiratory Distress Syndrome (Hyaline Membrane Disease); anemia: These and dozens of other conditions and illnesses are now treated in Cuba using domestically manufactured products that have successfully completed trials according to international GCP standards. Of the 857 medicines on Cuba’s Basic Drug List (medicines approved for use in the national health system), 569 are produced domestically. The introduction of these and other products into Cuba’s universal health system is having measurable impact on indicators and clinical protocols; contributes to import substitution (a major sustainable development goal in Cuba’s volatile and sluggish economy); and bolsters hard currency earnings as more of these products are tested, approved and marketed overseas.
Following Phase IV trials of recombinant interferon, a national therapeutic program was implemented to combat respiratory papillomatosis in children and adults leading to lower incidence of recurring papillomas. In clinical trials combining alpha recombinant interferon with Ribavirin, a therapeutic treatment for chronic Hepatitis C, 30% of participants—both adults and children—were cured. Phase IV trials to treat chronic myeloid leukemia and multiple myeloma revealed biopsy quality control and other procedural problems, spurring new protocols: For instance, biopsies are now analyzed at the Hematology Institute and to receive treatment, patients must be in remission. Administering hyperimmune inmunoglobulin in combination with a Cuban-produced vaccine in maternity hospitals across the country to babies born to mothers with Hepatitis B has resulted in 99% protection from this disease. For more than 15 years, Surfacén (recipient of the 2007 WIPO Gold Medal) has been administered to newborns with hyaline membrane disease in neonatal services nationally, contributing to a reduction in infant mortality from this condition of 0.2 per 1000 live births. Heberfastline, a pregnancy test developed by CIGB, has enabled Cuba to substitute similar tests previously imported from France.[3]
ONGOING TRIALS & RECENT DEVELOPMENTS
Accelerated research and development by Cuba’s biotechnology and medical diagnostic sectors, combined with more efficient and higher-quality clinical trials, are making their mark throughout the national health system. Of the 265 Phase I-IV clinical trials approved since 2012, treatments for several types of cancer (including head, neck, lung, brain, breast and esophageal), plus a novel diabetic foot ulcer (DFU) therapy, have been approved for use in Cuba. The results are also generating increased global interest in Cuban biotech and pharmaceutical products, with more scientific exchanges, technology transfer agreements and joint ventures on the table.[6] In 2012, BioCubaFarma was founded, Cuba’s corporate entity overseeing technology transfer, manufacturing contracts and joint venture agreements with foreign institutions.
Cancer—also a leading cause of death in Cuba—is high on the list of Cuban research priorities, development of new therapies now constituting the bulk of the biotech product pipeline. Indeed, 22% of clinical trials in Cuba have been conducted on cancer treatments. Nimotuzumab, a humanized monoclonal antibody to antiepidermal growth factor receptor developed at the Molecular Immunology Center (CIM) in Havana, has been in trials for 15 years, which thus far have shown it to be safe and effective in prolonging survival and controlling several types of cancer, by inhibiting tumor growth and reducing tumor size. CECMED first registered its use in Cuba in 2005 to treat advanced head and neck tumors; in 2007, it was approved for treatment of malignant glioma in children and adults.
Results of a clinical trial (conducted in 2005–2007) among Cuban pediatric patients with progressive or recurring primary brain tumors found nimotuzumab provided disease control with prolonged stabilization in 64% of participants (recovery of neurological functions and improved quality of life were also noted in this group). Survival for these children was 82% at 6 months and 64% at 1 year, with a median survival time of 19 months.[7] More recently, nimotuzumab has been approved in Cuba for treatment of head, neck, esophageal and pancreatic tumors, and is the subject of 27 clinical trials in Cuba and internationally for treatment of 11 different types of cancer. To date, nimotuzumab has undergone some 60 clinical trials worldwide, involving more than 1000 participants in contexts as diverse as Japan, Brazil, Canada, Germany, India and the Philippines; its use has thus far been approved in two dozen countries.[8]
Two therapies—Heberprovac, developed by CIGB to treat advanced prostate cancer and CIMAvax-EGF, developed by CIM to treat non-small cell lung cancer (NSCLC)—are in ongoing trials. Heberprovac, which targets adenocarcinoma prostate tumors by inhibiting testosterone growth, began Phase III clinical trials in Cuba in 2015 to test its efficacy compared to Zoladex, a leading treatment for this and other types of cancer internationally. Early results show longer survival times and improved quality of life.[9]
CIMAvax-EGF Phase IV trials (concluded in 2015), held across 61 sites and involving over 1000 participants, are among the country’s most “complex clinical trials conducted to date, involving the greatest number of clinicians and sites, and the first to be held in primary care services nationwide,” says Gladys Jiménez, Director of Data Management and Statistics at CENCEC. Developed to stimulate immunological response by releasing effector antibodies in adult patients with advanced NSCLC, treatment with CIMAvax-EGF has resulted in an average survival rate of more than 20 months in participants vaccinated with the therapy, with a significant difference (p <0.05) registered in patients under 60 years of age.[10,11] Over 5000 Cuban patients have been treated with CIMAvax-EGF and its application was “recently extended to over 160 sites. This year, CIMAvax-EGF will be available in primary care services across the country,” according to CENCEC Deputy Director Mayté Amoroto. Access to this vaccine is important in a country where “the best tobacco in the world is grown and smoking is a major risk factor,” says CIM Clinical Researcher Dr Camilo Rodríguez.“ For a long time, lung cancer has been the number one cause of cancer deaths in Cuba and treating it has been a priority of our health system for over 30 years.”
Non-small cell lung cancer is also the number one cause of cancer deaths in the United States and around the world (5,000 patients in other countries have been treated with CIMAvax-EGF), and ongoing Cuban and international trials are drawing the attention of scientists, clinicians and patients in the United States. Says CIM Project Manager and biologist Gryssell Rodríguez, “since CIMAvax-EGF was approved for use by Cuban regulatory authorities, we’ve received hundreds of treatment requests from patients around the world—including the USA.” Roswell Park Cancer Institute, based in Buffalo, NY, signed an agreement with CIM in June 2015 to begin Phase I CIMAvax-EGF clinical trials in the United States; the US Treasury Department has granted the license necessary for Roswell Park to “take part in all transactions necessary for collaborative research with the Centro de Inmunología Molecular (CIM)” to pursue trials testing CIMAvax-EGF, nimotuzumab, racotumomab (another lung cancer vaccine) and two other Cuban anticancer immunotherapies—[12]the first step in a long process before trials can actually begin. European trials of CIMAvax-EGF are already under way.
Timeline of Clinical Trials in Cuba
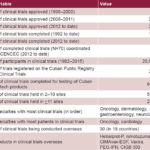
As of 2015, Cuban biotech products were exported to 49 countries and there were half a dozen products in 30 clinical trials in 18 countries.[13] Nevertheless, the product in the nation’s portfolio making the most headlines is Heberprot-P (winner of the 2011 WIPO gold medal). A novel treatment for DFU, it relies on recombinant human epidermal growth factor (EGF) and has treated over 250,000 patients in Cuba and 25 other countries since its initial registration for use in Cuba in 2007.[13] The application of Heberprot-P in Cuba has significant implications, given that diabetes mellitus is one of the top 10 causes of death on the island, with 15,000 new cases of DFU annually; up to 30% of these Cubans risk amputation.[14]
Clinical trials of Heberprot-P in Cuba revealed some unprecedented results that led to altered treatment protocols and in turn, improved clinical outcomes. Phase I trials conducted between 2001 and 2002 provided evidence regarding the safety and efficacy of injecting recombinant EGF directly into chronic, complex wounds (rather than applying it topically, an approach pursued for over three decades by researchers and clinicians worldwide). These trials were followed by others, including a double-blind, placebo-controlled Phase III multicenter trial conducted throughout Cuba, applying EGF via intralesional infiltration. Trials proceeded through Phase IV pharmacosurveillance with 95% of the 1835 participants experiencing only mild to moderate adverse effects,[14] while reducing relative amputation risk by 70%.[15]
“Without doubt, the product generating the most interest overseas is Heberprot-P. There’s a lot of demand, in Europe especially, where it’s poised to enter the market, and in the USA as well,” says Carlos Manuel García, Deputy Director of CENCEC. Over 70,000 amputations are performed on Americans annually due to DFUs, and half these patients die within five years of amputation. Under US embargo regulations, American companies must seek approval for two separate licenses to complete the regulatory and marketing process for such Cuban-developed medications: first requesting a license from the US Department of the Treasury’s Office of Foreign Assets Controls to conduct trials, and upon FDA approval, submitting a second license application for marketing. Critics maintain that the embargo regulations unduly delay entry into the normal regulatory channels.
CHALLENGES REMAIN
Clinical trials in Cuba are beset by challenges faced by all biotechnology and pharmaceutical developers, including the length of time between product concept and development, and when it finally becomes available to patients. Inherent in this challenge is the massive investment needed to bring a treatment successfully to market. Identifying and enrolling eligible participants in large-scale clinical trials is another difficulty Cuba shares with developers and CROs around the world. “Inclusion criteria are very selective and identifying enough candidates who fit trial criteria can be problematic. We’ve had experience with two promising products—one for asthma, the other for migraines, two conditions with high prevalence in Cuba—but even so, we were unable to identify enough candidates to conduct trials. No patients means no trials. This is why we’re seeing a trend towards more multinational trials—to broaden the universe of possible candidates,” says head of CENCEC’s International Cooperation Division, Julián Rodríguez. One advantage, points out Rodríguez, is that Cubans have historically been most willing to participate, once eligible for trials.
In Cuba, additional difficulties can arise once a trial has been approved by the relevant regulatory agencies. All material resources and inputs necessary to carry a clinical trial to its conclusion must be guaranteed before the trial is run, and herein lies the challenge: chronic resource scarcity is a reality on this cash-strapped island, where the US embargo is also still in effect. Inability to ensure resources can hamstring developers anxious to test their treatments. Another challenge observed during the monitoring and evaluation of early clinical trials was subjectivity on the part of health personnel and associated deviations from established protocols,[5] complicated by the complexities of coordinating trials across multiple sites. The infrequency with which Cuban scientists publish in high-impact, peer-reviewed journals has also been identified as a limitation, since publishing serves to disseminate findings, build on results and foster scientific exchange.[16]
However, the greatest challenge according to Cuban specialists, is lack of certain technology. Whereas in other contexts electronic medical records and remote data transmission are the norm, in Cuba, many clinical records are still kept the old-fashioned way—starting with paper and pen, maintained in bound books. Clinical trial data are recorded in similar books and then entered in the central database of the sponsoring agency—whether CENCEC or the research institute coordinating the trial. During the infrastructure reorganization undertaken in the early 1990s, Cuba incorporated available technology and introduced practical training for the clinical trials program. New positions were created including data managers, statistical analysts and auditors, to maximize data quality; double data entry (on two separate computers) is now standard. Statistical collection and databases themselves undergo independent quality assessments; once the data are recorded, the database is subject to another quality control check. Nevertheless, when the original data are written in longhand, across multiple sites, errors can be introduced. There is also the question of storing original documentation. “We’ve been conducting clinical trials for 25 years,” says CENCEC Deputy Director García. “We would need an entire building to house all the hard-copy records. Also, what happens when the database manager encounters a number and can’t decipher whether it’s a six or seven the clinician has written? There are clear advantages to electronic files—they’re more cost-effective, data can be monitored and shared with the click of a mouse, and they can reduce errors.”
Recording and sharing data electronically are tools Cuban researchers are keen to implement. MINSAP is exploring a pilot project at Havana’s Hermanos Ameijeiras University Hospital to digitize medical records and doctors’ signatures. “This reference hospital conducts many clinical trials and electronic medical records will help us build experience and efficiency. It’s a process and involves significant investment, but it will eventually benefit our clinical trial work,” says Dr Sandra Álvarez, head of CENCEC Quality Control Management. Cuba’s international partners also take into account technological challenges when proposing collaboration, analyzing what software, connectivity, remotely accessible databases and the like will be needed to carry out quality clinical trials. “Their assessment allows development of parallel projects—the actual trial, but also IT updating, support and training,” says CENCEC’s Julián Rodríguez. “We’re just starting to explore this type of cooperation, and certainly having the proper technology in place will accelerate the possibility of conducting international clinical trials in Cuba.”
CONCLUSIONS
Along with Brazil and India, Cuba’s biotech industry is considered one of the Global South’s “big three.” To maximize the potential of this sector and its products, Cuba needed a quality clinical trials infrastructure based on international GCP standards. This prompted implementation of uniform requirements for trial design and monitoring, updating and enforcing ethical guidelines, training specialists and improving quality control. Once established, the new structure provided the foundation for testing domestically produced therapies, vaccines and medical technologies, while positioning Cuba to compete in the global marketplace. The clinical trials strategy now in place is helping the country improve domestic health indicators and delivery of services, as well as cultivate greater options for international cooperation by building confidence in its clinical trials capabilities.
References

- For a detailed explanation of this strategy, see “Connecting Science to Population Health: The ‘Closed Loop’ Approach,” by Augustín Lage, MEDICC Rev. 2007 Fall;9(1):48.
- For complete list of Cuban biotech WIPO Gold Medal Award winners, see http://www.ocpi.cu./sites/default/files/archivos_a_vincular/medallasoro.pdf
- Pascual MA, Jimenez G, Torres A, Fors MM, López I. Cuba’s National Clinical Trials Coordinating Center: Emergence, Evolution and Main Results. MEDICC Rev [Internet]. 2010 Apr-Jun [cited 2016 Jun 21];13(1):46–51. Available from: http://www.medicc.org/mediccreview/index.php?issue=15&id=184&a=va
- Ministry of Public Health (CU). Anuario Estadístico de Salud, 2015. Havana: Ministry of Public Health (CU). 2016. Spanish.
- Pascual MA. Los ensayos clínicos en Cuba: Impacto en la biotecnología y en la salud pública [unpublished Master’s thesis]. [Havana]: [place unknown]; 2013. Spanish.
- 6.World Health Organization. Cuban experience with local production of medicines, technology transfer and improving access to health. Geneva: World Health Organization; 2015.
- Suarez G, Cabanas R, Zaldívar M, Garnier T, Iglesias B et al. Clinical Experience with Nimotuzumab in Cuban Pediatric Patients with Brain Tumors, 2005 to 2007. MEDICC Rev [Internet]. 2009 Summer [cited 2016 17 Jul]; 11(3):27–33. Available from: http://www.medicc.org/mediccreview/index.php?issue=9&id=102&a=va
- Granma Internacional. Clinical trials of promising Cuban monoclonal antibody expanded. Granma [Internet]. 2016 Apr 7 [cited 2016 Jun 21]. Available from: http://en.granma.cu/cuba/2016-04-07/clinical-trials-of-promising-cuban-monoclonal-antibody-expanded
- EFE. Cuba begins 3rd phase of clinical trials of treatment for prostate cancer. EFE [Internet] 2015 Aug 19 [cited 2016 Jul 4]. Available from: http://www.efe.com/efe/english/life/cuba-begins-3rd-phase-of-clinical-trials-treatment-for-prostate-cancer/50000263-2691573
- Rodríguez PC, Rodríguez G, González G, Lage A. Clinical Development and Perspectives of CIMAvax-EGF, Cuban Vaccine for Non-small-cell Lung Cancer Therapy. MEDICC Rev [Internet]. 2010 Winter [cited 2016 Jun 4]; 12(1):17–23. Available from: http://www.medicc.org/mediccreview/index.php?issue=11&id=128&a=va
- Gorry C. Cuba’s Complex Respiratory Picture. Lancet Resp Med. 2015 Sept; 3.
- Lee K. A small boat in a large ocean: One US cancer center’s ongoing journey to bring Cuban cancer vaccines to American patients [PowerPoint presented at the 14th Edition of the Series of Conversations: Cuba in US Foreign Policy]; Havana: 2015 Dec 16.
- Raices Pérez-Castañeda M. Papel de la organización superior de desarrollo empresarial BioCubaFarma en la concreción dentro de la sociedad cubana de proyectos biotecnológicos orientados al beneficio social [PowerPoint]; 2015. Spanish.
- Berlanga J. Fernández JI, López E, López PA, del Río A, et al. Heberprot-P: A Novel Product for Treating Advanced Diabetic Foot Ulcer. MEDICC Rev [Internet]. 2013 Jan [cited 2016 Jul 3]; 15(1):11–5. Available from: http://www.medicc.org/mediccreview/index.php?issue=23&id=287&a=va
- Reed GA. Let’s open the door to Cuba and its promising diabetes treatments. SF Chronicle [Internet]. 2016 Mar 17 [cited 2016 Jul 16]. Available from: http://www.sfchronicle.com/opinion/article/Let-s-open-the-door-to-Cuba-and-its-promising-6921055.php
- Arrencibia-Jorge R, Corera-Alvarez E, Chinchilla-Rodríguez Z, de Moya-Anegón F. Scientific output of the emerging Cuban biopharmaceutical industry: a scientometric approach. Scientometrics. 2016 May. DOI: 10.1007/s11192-016-2023-1





