ABSTRACT
Public health systems face the contradiction of skyrocketing cancer incidence and cancer drug prices, thus limiting patient access to more effective treatments. The situation is particularly dire in low- and middle-income countries. We urgently need consensus on the main determinants of this problem, as well as specific, effective and feasible solutions.
Analysis of available data reveals that the problem has reached its current magnitude only recently and is not related to the growing complexity of drug production technology, but rather to corporate profits and the failure of market mechanisms to allocate resources based on health needs.
Despite the obstacles, there is ample room for effective intervention: joint price negotiations, cost transparency, greater support for creation of manufacturing capacity, and regulatory measures that facilitate introduction of generic and biosimilar drugs and reduce intellectual property barriers to better use of flexibilities in the Agreement on Trade-Related Aspects of Intellectual Property Rights.
Such actions will not be effective if there is no consensus around them, or if low- and middle-income countries act in isolation. This is precisely where international organizations must intervene.
KEYWORDS Public health, price, cancer drugs, inequality, less-developed countries, developing countries, Cuba
INTRODUCTION
Cancer is one of the main causes of mortality worldwide, with 8.8 million deaths in 2015. In 2012, there were 14 million new cases, a number expected to grow by 70% in the coming two decades.[1]
The economic impact of cancer is substantial and growing, with US$1.16 trillion in treatment costs in 2010.[2] According to Prasad, at current prices, providing drugs to all patients with cancer, as the treatment paradigm for cancer indicates, would lead all nations, even the most prosperous, to bankruptcy.[3]
This paper reviews the possible causes and repercussions of rising prices for cancer drugs and offers suggestions for addressing these challenges in low- and middle-income countries.
DEVELOPMENT
Rising prices are a recent problem Surgery and radiotherapy were the main therapeutic options until antitumor drugs appeared in the mid-twentieth century. Availability of these drugs is now a determining factor in treatment quality. With implementation of the Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS) in 1995, cancer treatment costs increased. Prasad found that the average cost of these treatments surpassed average family income in 2004 and were twice average family income in 2014.[3]
The impact of unreasonably high prices for lifesaving drugs is similar to that of price gouging for necessities in isolated areas experiencing natural disasters.[4] The UN Sub-Commission on the Promotion and Protection of Human Rights recognized that, although States have the primary responsibility for promoting, respecting and protecting human rights, transnational corporations “are also responsible for promoting and securing . . . human rights.”[5]
IMPORTANCE High prices for cancer drugs are a stumbling block to the goals of ensuring universal drug coverage and access to better treatments. This article reviews the possible reasons for and repercussions of rising cancer drug prices and offers suggestions for how developing countries can respond.
Annual treatment costs per patient for 12 of 13 cancer drugs approved in the USA in 2012 are over US$100,000.[6] Furthermore, according to an analysis of 71 cancer drugs approved from 2002 through 2012 for treatment of patients with solid tumors, median overall survival was 2.1 months, while progression-free survival was 2.3 months.[7] In another analysis of 47 pharmaceuticals approved by the US FDA in 2014–2016, only 9 (19%) met the significant clinical benefit standard with regard to overall survival established by the American Society of Clinical Oncology.[8] In addition, in an analysis of 226 randomized trials, only 70 (31%) met the threshold for significant clinical benefit proposed by the European Society for Medical Oncology.[9] These marginal results come from randomized controlled trials that supported regulatory approval of these drugs. These therapies’ benefits are even lower in the general population (i.e., outside research settings), which is generally older with more comorbidities than patients recruited into clinical trials.
A systematic examination of cost–effectiveness of drugs for treatment of hematologic cancers found that only 9 of 24 drugs analyzed (37.5%) had incremental cost–effectiveness ratios below the benchmark of US$50,000 per quality-adjusted life year.[10]
Low- and middle-income countries have it worse Age is the strongest risk factor for cancer. The numbers of older adults worldwide are increasing; two thirds of the population aged ≥60 years live in low- and middle-income countries. In 2015, 47.2% of persons aged >80 years were living in developed countries and 52.8% in low- and middle-income countries. By 2050, the latter proportion is expected to increase to 70.6%.[11]
Although cancer is frequently identified as a problem of the industrialized world, 70% of cancer deaths occur in low- and middle-income countries. In 2017, only 26% of these countries reported having pathology services in the public health sector, where the greatest share of patients receive care, if they receive care at all. Fewer than 30% of these public health systems had cancer treatment services, contrasting with over 90% in high-income countries. Only one in five low- and middle-income countries compiles data needed to support cancer policies. In low- and middle-income countries, even though cancer drug prices might be lower than in developed countries, treatments are less affordable due to citizens’ lower average purchasing power.[12]
It could be argued that high drug costs are not a problem in countries with universal health coverage (of the 194 UN-member countries, only 36% guarantee the broad right to health in their constitutions),[13] but with current drug costs, budget constraints become inevitable, even in countries with the political will to assume the financial burden of citizens’ treatment needs. Cancer drugs are simply unaffordable in many countries, and if current pricing trends continue, it is only a matter of time before they become unaffordable for all countries. Rising expenditures on cancer drugs can also siphon off funds from drugs to treat other types of illnesses, which risks endangering the health of the population as a whole.
Individual nations have very limited bargaining power to bring down prices, due to the modest size of their markets.[7] Therefore, capacity for collective bargaining with the pharmaceutical industry is becoming increasingly important.
The problem is profit, not technological complexity Managing drug development, production and trade in a globalized world leads industry to evade the problem of accessibility, which it frames as a function of “market failure.” A determining factor in price hikes is that pharmaceutical and biotechnology companies tend to be private, and they prioritize amassing wealth at the expense of the public interest in obtaining access to drugs at affordable prices.
The industry argues that unfettered price-setting is crucial to incentivizing innovation, since it allows for recovery of investments in product development. However, evidence suggests that research funded with public money has a direct role in innovation of 10%–40% of new drugs and that the indirect role of science funded with public funds is even more substantial.[14–16]
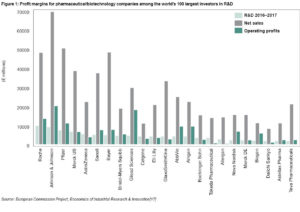
In the absence of corporate transparency and independent auditing of their accounting records, it is difficult to accurately calculate drug development costs and to what extent public or private funders cover these costs. Pharmaceutical corporations continue reporting annual profits of up to €20 billion,[17] suggesting that revenues substantially exceed expenditures. Evidence indicates that more money is spent on marketing than on research and development (R&D), generating high profit margins for the few companies that control the bulk of the market, most of which are located in only nine countries (Figure 1).[17]
Furthermore, pharmaceutical companies use various strategies to delay or prevent introduction of generic or biosimilar drugs. Patent holders often pay potential manufacturers to delay introduction of generically equivalent drugs.[18] Another trend includes FDA Risk Evaluation and Mitigation Strategies (REMS) programs.[19] The FDA requires prescribers and patients to be informed of possible risks and benefits associated with a medication’s use. Some drug manufacturers patent their own REMS program, and later deny access to generics manufacturers that wish to conduct bioequivalence studies. Major pharmaceutical corporations have responded to expiration of their patents by pressuring regulatory agencies to implement increasingly stringent controls, raising the entry bar for new competitors. These strategies have been successful for corporate interests, but not for the needs of public health systems or the patients they serve.
No discussion of generic and biosimilar drugs would be complete without considering intellectual property and its relationship to market exclusivity. Patents determine the exclusivity that businesses enjoy of exercising a monopoly under protection of law.[3] In an analysis of 437 top-selling drugs, market exclusivity lasted an average of 12.5 years; for drugs categorized by the FDA as hematology/oncology drugs, this period was 14.3 years.[20]
Elements that shore up drug prices include a dearth of competitors and an overall lack of a relationship among a given drug’s price, sales volume and clinical performance. In fact, lack of competitors and bargaining power made cancer drug prices in the USA among the highest in the world, increasing by 10% annually between 1995 and 2013, far exceeding the USA’s average inflation rate for the period.[21]
The pharmaceutical industry has defended these high prices by arguing high development costs due to the large clinical studies required to obtain regulatory approval. However, in this new era of personalized medicine, cancer-fighting drugs are often genotype-selective, which increases their success rate with more precise patient selection. Consequently, it is estimated that approval of these drugs will require less costly clinical trials. The European Medicines Agency has launched an adaptable licensing program that enables companies to obtain marketing authorization on the strength of well-designed trials based on biomarkers.[22] Other regulatory agencies might follow this example.
However, major challenges still need to be overcome regarding efficiency in the overall drug development process. For example, there are currently 803 clinical trials of therapeutic candidates with immune checkpoint inhibitors, which are expected to enroll more than 166,000 patients.[23] Enormous redundancy in these studies comes from many companies conducting similar trials with comparable drugs, but not sharing their data, all to protect commercial interests.
How is the international community dealing with these challenges? WHO has developed initiatives to resolve the conflict between market-driven approaches and public health interests. However, such efforts are still insufficient to sustainably address health priorities. As part of these initiatives, WHO publishes Model Lists of Essential Medicines (EML) aimed at meeting priority population health care needs and helping establish the principle that some drugs are more useful than others.[24] Several controversies have arisen around the list, due to its influence in determining reimbursements in drug procurement programs in many developing countries. For example, there was a time when HIV treatments, which save lives, were left off the EML because their purchase was not cost-effective. As a result, countries such as the BRICS (Brazil, Russia, India, China and South Africa) began to produce them even though their patents had not expired, which is how they came to be included in the EML.
However, the 20th edition of the EML (March 2017) still does not include products for cancer treatment on its core list. Cancer drugs registered several years ago remain on the complementary list, which includes drugs that require specialized facilities or care, or are very expensive. At present, the EML only includes three biological products.[24]
CONTROVERSIAL BUT POSSIBLE SOLUTIONS
Change the rules for price negotiations between governments and industry If nations had greater capacity to negotiate prices as a group, entering negotiations with consensus-based prices could have worldwide repercussions. To facilitate this process, WHO could establish an international open database of drug prices in all countries where they are commercially available. This should be a requirement for all drugs in the EML and should be complemented by introduction of a new category: medicines that would be classified essential if they were available at affordable prices.
An interesting example of this approach, although still controversial, is that of the UK National Institute for Health and Care Excellence, which has proposed provisional funding for use of certain drugs within the cost–effectiveness threshold and gathering data on their use to more accurately gauge their cost–effectiveness.[25]
Make drug development costs transparent The pharmaceutical industry argues that R&D expenses are a key component of the high cost of cancer drugs. However, R&D expenses associated with introduction of a new drug are for the most part unknown or speculative. In the USA there are initiatives to make composition of drug prices more transparent, such as Vermont’s 2016 cost-transparency legislation.[26] This trend expresses the will to put an end to legal monopolies on products when prices are excessive. Such laws require manufacturers to provide details on R&D, manufacturing and marketing costs, as well as probable clinical benefits and prices set in other countries.[3]
Introduce rigorous programs to develop biomarkers Regulatory agencies should set standards for drug approval using validated and clinically useful biomarkers for patient selection. This would decrease costs by reducing the number of patients treated with drugs that do not improve survival or quality of life. In addition to discovering innovative pharmaceuticals, efforts should be aimed at repurposing drugs with expired patents, by looking for new indications linked to effective biomarkers that predict response.[3]
Speed up introduction of generic and biosimilar drugs Generic drugs are 80%–85% less expensive than their brand name equivalents. Hence, there is considerable public interest in ensuring their early and safe introduction, especially in oncology. It would be desirable to require patent holders to provide samples of drugs to manufacturers of generics and biosimilar drugs, to facilitate the studies needed to gain regulatory approval. This should also be extended to REMS programs and to knowhow, which are currently kept confidential and are required for health registration of both generic and biosimilar drugs.
Expand and deepen use of TRIPS flexibility The Doha Declaration on the TRIPS Agreement affirmed that it “can and should be interpreted and implemented in a manner supportive of WTO members’ right to protect public health and, in particular, to promote access to medicines for all.”[27]
A recent study found that TRIPS flexibility mechanisms were used by 89 countries from 2001 through 2016, most (56.8%) in the form of compulsory licenses or public noncommercial use licenses.[28] Although the authors concluded that TRIPS flexibilities have been applied more often than commonly assumed, expanded and more thorough implementation is still needed.
In 2016, the report of the UN Secretary-General’s High-Level Panel on Access to Medicines stressed: “Countries have the right to authorize and issue compulsory licenses. This right is explicitly safeguarded in leading intellectual property and trade treaties and in national laws.” However, the same report also recommended better coordination among UN interagency working groups to “ensure greater coherence in the advice and support to governments and other stakeholders,” clearly recognizing that the problem is complex and far from being resolved.[29]
WHO could advocate for mechanisms that maximize innovation, promote access and introduce creation of manufacturing capacity in developing countries through technology transfer incentives. Geographic concentration of manufacturing capacity in only a few countries does not contribute to price controls or affordability.
Increasingly, WHO has been leading stakeholder discussions aimed at designing solutions to the issue of high drug prices. To develop an effective policy framework, WHO must work with other UN system agencies, such as the WTO, the World Intellectual Property Organization and the UN High Commissioner for Human Rights.
CONCLUSIONS
Rising cancer incidence, which increasingly affects nations of the Global South, is incompatible with the global trend of increasing cancer drug prices. Solutions depend on collaboration to enhance the right to treatment for cancer patients, irrespective of their country of origin or socioeconomic situation.
The pharmaceutical industry’ economic power makes the struggle to promote policies to reduce prices more difficult. Developing countries are particularly defenseless, unless they can work in concert to boost their bargaining power. UN institutions can play a decisive role by providing a more coherent policy framework that brings incentives for innovation and trade into line with efforts to ensure basic human rights, including access to medicines, wherever the market fails to do so.
References

- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015 Mar 1;136(5):E359–86.
- Union for International Cancer Control. The Economics of Cancer Prevention and Control. Data Digest [Internet]. Geneva: Union for International Cancer Control; 2014 [cited 2018 Oct 5]. 8 p. Available from: http://issuu.com/uicc.org/docs/wcls2014_economics_of_cancer_final?e=0
- Prasad V, De Jesús K, Mailankody S. The high price of anticancer drugs: origins, implications, barriers, solutions. Nat Rev Clin Oncol. 2017 Jun;14(6):381–90.
- Gross A. The global economic cost of cancer: improving outcomes and cost by reducing international barriers to care. Loy U Chi Int’l L Rev [Internet]. 2015 [cited 2018 Apr 10];12(2):231–47. Available from: http://lawecommons.luc.edu/lucilr/vol12/iss2/7
- United Nations [Internet]. New York: United Nations; 2003. Commentary on the Norms on the Responsibilities of Transnational Corporations and Other Business Enterprises with Regard to Human Rights, U.N. Doc. E/CN.4/Sub.2/2003/38/Rev.2; 2003 [cited 2018 Apr 10]. Available from: http://hrlibrary.umn.edu/business/commentary-Aug2003.html
- Mailankody S, Prasad V. Five years of cancer drug approvals: innovation, efficacy, and costs. JAMA Oncol. 2015 Jul;1(4):539–40.
- Fojo T, Mailankody S, Lo A. Unintended consequences of expensive cancer therapeutics – the pursuit of marginal indications and a me too mentality that stifles innovation and creativity: the John Conley Lecture. JAMA Otolaryngol Head Neck Surg. 2014 Dec;140(12):1225–36.
- Kumar H, Fojo T, Mailankody S. An appraisal of clinically meaningful outcomes guidelines for oncology clinical trials. JAMA Oncol. 2016:2(9):1238–40.
- Del Paggio JC, Azariah B, Sullivan R, Hopman WM, James FV, Roshni S, et al. Do Contemporary Randomized Controlled Trials Meet ESMO Thresholds for Meaningful Clinical Benefit? Ann Oncol. 2017:28(1):157–62.
- Chhatwal J, Mathisen M, Kantarjian H. Are high drug prices for hematologic malignancies justified? A critical analysis. Cancer. 2015 Oct 1;121(19):3372–9.
- United Nations Department of Economic and Social Affairs Population Division. World Population Ageing Report [Internet]. New York: United Nations; 2015 [cited 2018 Apr 10]. 149 p. Available from: https://www.un.org/en/development/desa/population/publications/pdf/ageing/WPA2015_Report.pdf
- Goldstein DA, Clark J, Tu Y, Zhang Y, Fang F, Goldstein RM, et al, editors. Global differences in cancer drug prices: A comparative analysis. J Clin Oncol [Internet]. 2016 [cited 2018 Feb 10];34(Suppl 18). Available from: http://ascopubs.org/doi/abs/10.1200/JCO.2016.34.18_suppl.LBA6500#
- Heymann J, Cassola A, Raub A, Mishra L. Constitutional rights to health, public health and medical care: the status of health protections in 191 countries. Glob Public Health. 2013 Jul;8(6):639–53.
- Galkina Cleary E, Beierlein JM, Khanuja NS, McNamee LM, Ledley FD. Contribution of NIH funding to new drug approvals 2010–2016. Proc Natl Acad Sci U S A. 2018 Mar 6;115(10):2329–34.
- Mazzucato M. The Entrepreneurial State – Debunking Public vs. Private Sector Myths New York: Anthem Press; 2013 Jun 10. 266 p. ISBN 978-0-857282-52-1.
- Jacobs M, Mazzucato M. Rethinking Capitalism: Economics and Policy for Sustainable and Inclusive Growth. New Jersey: Wiley-Blackwell; 2016 Jul. ISBN: 978-1-119-12095-7.
- IRI – Economics of Industrial Research and Innovation [Internet]. Seville: IRI; c2018. Data World 2500. R&D ranking of the world top 2500 companies; 2017 [cited 2018 Apr 10]. Available from: http://iri.jrc.ec.europa.eu/scoreboard17.html#close
- Shah S, Silva MA, Malloy MJ. Are reverse payments and pay-for-delay settlements business as usual or an anticompetitive practice? Nat Biotechnol. 2016 Jul 12;34(7):716–9.
- Finch AC, Himes JL, Hollywood M, Pérez M, Reiss W, Sedlak L, et al. Antitrust plays whack-a-mole as exclusion of competition by drug monopolists pops up again: Gaming the –REMS–. NYSBA NY Litigator [Internet]. 2016 Fall [cited 2018 Apr 10];21(2):9–18. Available from: http://www.nysba.org/Sections/Antitrust_Law/Events/2016/REMS_-_Nov_3/NY_Litigator_Article.html
- Wang B, Liu J, Kesselheim AS. Variations in time of market exclusivity among top-selling prescription drugs in the United States. JAMA Intern Med. 2015 Apr;175(4):635–7.
- Howard DH, Bach PB, Berndt ER, Conti RM. Pricing in the market for anticancer drugs. J Econ Perspect. 2015;29(1):139–62.
- Eichler HG, Baird LG, Barker R, Bloechl-Daum B, Børlum-Kristensen F, Brown J, et al. From adaptive licensing to adaptive pathways: delivering a flexible life-span approach to bring new drugs to patients. Clin Pharmacol Ther. 2015 Mar;97(3):234–46.
- Brawley L. With 20 agents, 803 trials, and 166,736 patient slots, is Pharma investing too heavily in PD-1 drug development? Cancer Lett [Internet]. 2016 Oct 7 [cited 2018 Apr 10];42(37):2–18. Available at: https://cancerletter.com/articles/20161007_1/
- World Health Organization [Internet]. Geneva: World Health Organization; c2018. Publications. Essential medicines and health products. WHO Model Lists of Essential Medicines. The 2017 Expert Committee on the Selection and Use of Essential Medicines; 2017 Aug [cited 2018 Apr 10]. Available from: http://www.who.int/medicines/publications/essentialmedicines/en/
- Grieve R, Abrams K, Claxton K, Goldacre B, James N, Nicholl J, et al. Cancer drugs fund requires further reform. BMJ. 2016 Sep 27;354: i5090.
- Sullivan T. Vermont: First State to Pass Pharmaceutical Cost Transparency Bill. Policy and Medicine [Internet]. [place unknown]: Policy &Medicine; 2018 [cited 2018 Apr 10]; [about 3 screens]. Available from: https://www.policymed.com/2016/05/vermont-first-state-to-pass-bill-on-pharmaceutical-cost-transparency.html
- World Trade Organization [Internet]. Geneva: World Trade Organization; c2018. Declaration on the TRIPS agreement and public health; 2001 Nov 20 [cited 2018 Apr 10]; [about 2 screens]. Available from: https://www.wto.org/english/thewto_e/minist_e/min01_e/mindecl_trips_e.htm
- t’Hoen EF, Veraldi J, Toebes B, Hogerzeil HV. Medicine procurement and the use of flexibilities in the Agreement on Trade-Related Aspects of Intellectual Property Rights, 2001–2016. Bull World Health Organ. 2018 Mar 1;96(3):185–93.
- World Health Organization [Internet]. Geneva: World Health Organization; c2018. Public health, innovation, intellectual property and trade. United Nations Secretary-General’s High-Level Panel on Access to Medicines; 2016 Sep [cited 2018 Apr 10]. Available from: http://www.who.int/phi/implementation/ip_trade/high-level-panel-access-med/en/
THE AUTHORS
Einard Blanco-García*, biotechnologist with a master’s degree in drug technology and quality control, CIMAB S.A., Havana, Cuba.
Nuris Ledón-Naranjo* (Corresponding author: nuris@cim.sld.cu), biologist with a doctorate in pharmaceutical sciences and master’s degrees in pharmacology and business administration, Molecular Immunology Center (CIM), Havana, Cuba.
Agustín Lage-Dávila, physician specializing in biochemistry with a doctorate in medical sciences. Founding director of CIM and CIMAB S.A.; now researcher and consultant, BioCubaFarma, Havana, Cuba.
*Einard Blanco-García and Nuris Ledón Naranjo made equal contributions to the manuscript.
Submitted: August 10, 2018 Approved: October 06, 2018 Disclosures: None




