INTRODUCTION
Type 1 diabetes (T1DM) is one of the most frequent chronic non-communicable diseases in children and adolescents globally and its incidence has increased dramatically in the last 50 years. It results from autoimmune destruction of more than 80% of pancreatic β cells, producers of insulin.[1]
The first detectable signs of emerging autoimmunity against pancreatic β cells appear during the preclinical phase. These include islet cell antibodies (ICA) and antibodies against four of the main autoantigens of pancreatic β cells: insulin (AAI), glutamic acid decarboxylase (AGAD), the intracellular portion of a tyrosine phosphatase protein related to the IA2 molecule (AIA2),[2] and the zinc cation transporter within the pancreatic islet β cells (AZnT8).[2,3]
However, what triggers the immune response against pancreatic β cells remains unexplained. Genetic factors doubtless have great importance in T1DM pathogenesis, but numerous studies, particularly of monozygotic twins, show that hereditary factors only explain 30% to 50% of disease susceptibility.[4] Hence, attention has focused on the search for environmental factors that can induce or potentiate the immune phenomenon. Enteroviruses are among these possibilities and there is still debate on whether exposure to them could be related to pathophysiological processes that prompt, accelerate or perpetuate pancreatic β-cell damage.
The Virus in Diabetes International Study Group (VIDIS) was established in 2007 to enhance international cooperation and promote development of multicenter studies on association between infections with enterovirus or other viruses and T1DM.[5] In 2008, Cuba joined VIDIS and established a virus and diabetes working group to support related collaboration and research.
In our opinion, the establishment of a possible relation between enterovirus exposure and T1DM should be based on studies that aim to answer three main scientific questions:
- Is enterovirus exposure associated with preclinical stages of autoimmunity against pancreatic cells?
- Is enterovirus exposure associated with clinical diabetes onset?
- What mechanisms could explain such associations?
Most of the evidence linking enteroviruses with T1DM pathogenesis has been obtained in European countries with high T1DM incidence (>25 cases per 100,000 population)[6] and low prevalence of circulating enterovirus (4%–14%).[7] In this paper we consider the possible role of enteroviruses in the natural history of T1DM by reviewing results from research in Cuba, where the pattern of T1DM incidence (2.9 cases per 100,000 population) and enterovirus prevalence (30%–40%)[8] is the inverse of Europe’s.[9]
LINES OF EVIDENCE
Autoantibodies associated with type 1 diabetes in enterovirus-infected subjects The first studies done in Cuba explored possible association between enterovirus infection and induction of humoral markers of pancreatic β-cell autoimmune destruction. To do this, we examined acute phase (5–6 days after symptom onset) and convalescent phase (29–35 days after symptom onset) sera obtained from children and adolescents infected during two large epidemics of meningoencephalitis caused by echovirus 16 (E16) and echovirus 30 (E30) in 2000 and 2001, respectively.[10–13] ICA levels were measured for all sera by modified indirect immunofluorescence with prolonged incubation. Levels of antibodies against the three main pancreatic β-cell autoantigens (AAI, AGAD and AIA2) were measured by radioimmunoassay.[14,15].
There was seroconversion to ICA, AGAD, AIA2 and AAI in 92.1%, 28.9%, 44.7% and 42.1%, respectively, of E16-infected individuals. In E30-infected individuals, there was seroconversion to ICA (87.5%), AGAD (37.5%) and AIA2 (12.5%). In neither case did infected individuals develop antibodies against other organs, such as thyroid anti-microsomal or anti-gastric wall antibodies. Antibodies against pancreatic β-cell antigens were not detected in study controls without viral infection.[10–13] During the 2001 epidemic, an adolescent was reported to have developed ICA and AIA2, as well as T1DM following E30 meningoencephalitis; curiously, the patient had a low diabetes risk genotype (DR2/DR7 and DQ2/DQ6).[11,16]
Moreover, the presence of high ICA titers (>40 Juvenile Diabetes Foundation, JDF, units) was demonstrated in E16 (25.7%) and E30 (42.8%) convalescent phase patients compared with the acute phase of the infection, in which high titers were not found for this antibody.[10–13] An increase in viral neutralizing antibodies was found to be related to presence of antibodies against several pancreatic autoantigens. Thus, presence of high titers of viral neutralizing antibodies (1/160) was demonstrated in 100% of individuals who developed the four antibodies studied (ICA, AGAD, AIA2 and AAI). In contrast, of those individuals who developed ICA as sole antibody, only one patient showed neutralizing antibodies at a dilution of 1:40 (Table 1). These results indicate the likelihood that severe pancreatic β-cell damage produced by viral infection causes exposure of pancreatic antigens and their recognition by the immune system, generating high ICA titers and an extended humoral response against several autoantigens. As described in the literature, appearance of these autoantibodies and their association with damage severity may be predictive of T1DM development.[17] In spite of the high proportion of children and adolescents with evidence of an autoimmune response against pancreatic β-cell antigens, persistence of autoimmunity generated during viral infection and clinical expression of diabetes is unlikely to occur in all individuals, since T1DM incidence in Cuba is low.[9]
Table 1: Antibodies against selected pancreatic autoantigens and relation to neutralizing antibody frequency and titers in convalescent phase of Echovirus 16 infection

AAI: anti-insulin antibodies AGAD: anti-glutamic acid decarboxylase antibodies AIA2: anti-tyrosine phosphatase antibodies ICA: islet cell antibodies Max: highest titer Min: lowest titer
Enterovirus infection at clinical onset of type 1 diabetes and during the prediabetic period Presence of enterovirus RNA was determined in newly diagnosed type 1 diabetics and those at high risk of developing the disease, such as first-degree relatives of type 1 diabetics, to discern whether enterovirus infection was associated with clinical onset of T1DM or presence of humoral markers of pancreatic autoimmunity.
Presence of enterovirus RNA in sera from type 1 diabetics at clinical onset of the disease (9/34, 26.5%) was significantly higher than in healthy control individuals (2/68, 2.9%; p = <0.001). Considering that enterovirus RNA in serum is an indicator of viremia (a period of ≤7 days), these results suggest a temporal association between enterovirus infection and clinical diabetes onset (Table 2).[12,13,18,19] Presence of enterovirus RNA was also associated with severe ketoacidosis at T1DM clinical onset.[18] Since most T1DM patients studied had ICA at diagnosis,[13] these individuals probably had lost a considerable amount of pancreatic β-cell mass by progressive autoimmune damage, but acute cytolytic damage to pancreatic islet cells caused by viral infection could have been the final precipitating event.
Higher prevalence of enterovirus RNA was detected in sera of first-degree relatives of ICA-positive type 1 diabetics (5/32, 15.6%) than in healthy controls (0/64, 0.0%; p = 0.003). Statistically significant differences were also found when ICA-positive and ICA-negative first-degree relatives were compared with respect to enterovirus RNA presence (1/62, 1.6%; p = 0.016) (Table 2), which suggests an association between enterovirus exposure and autoimmunity against pancreatic b cells.[18,19]
Table 2: Enterovirus RNA in newly diagnosed type 1 diabetics, first-degree relatives of type 1 diabetics, and control group
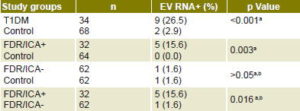
a Sarmiento[18] b Cubas-Dueñas[19] EV RNA+: enterovirus RNA positive FDR/ICA+: first-degree relatives of ICA-positive type 1 diabetic FDR/ICA-: first-degree relatives of ICA- negative type 1 diabetic ICA: islet cell antigen T1DM: Newly diagnosed type 1 diabetic
These results indicate that enteroviruses may exacerbate pancreatic β-cell damage and thus contribute to activation of a larger number of memory clones, perpetuating the autoimmune process. Given the multifactoral nature of T1DM, the trigger event for pancreatic β-cell autoimmunity could be toxic, nutritional or psychosocial in nature, or even infection by another enterovirus.[2,13,20]
Mechanisms for development of enterovirus-mediated autoimmunity against pancreatic β cells Analysis of findings presented here supports the hypothesis that enterovirus infection triggers, perpetuates or precipitates pancreatic β-cell autoimmune destruction. However, the mechanism by which enteroviruses can mediate an immune response directed against pancreatic b cells is not well understood. It has been postulated that enteroviruses could replicate in pancreatic b cells and cause their direct destruction as a result of acute or persistent cytolytic infection.[21] On the other hand, it is possible that activation of innate and adaptive immune responses to viral infection could cause a proinflammatory environment with cell toxicity and release of pancreatic β-cell autoantigens, hitherto unrecognized by the immune system, provoking an autoimmune response (fertile field theory). It has also been proposed that enteroviruses could cause indirect destruction of pancreatic β cells through nonspecific activation of autoreactive cells and mimicry or molecular similarity between pancreatic autoantigen determinants and viral elements.[21]
Among these proposed mechanisms, our studies focused on molecular mimicry. We assessed antibody induction against the main autoantigens of human pancreatic b cells in rabbits inoculated with different enterovirus serotypes; the rabbit was chosen as the experimental model in these studies because enteroviruses do not replicate in rabbit cells,[22] ruling out the possibility of a humoral immune response resulting from damage to the animal’s pancreatic b cells. Antibodies against human GAD65 were detected in sera of rabbits inoculated with echoviruses 9, 11, 16, 30; and coxsackieviruses B1, B3 and B5. It is interesting to point out that the serotypes for which GAD65 antibodies were found corresponded to enterovirus B species, for which a 6-amino acid homology between viral P2-C protein and human GAD65 has been described.[23] The fact that human GAD65 antibodies were found in rabbit hyperimmune sera prepared against E16 and E30 epidemic strains is particularly noteworthy. This finding suggests that molecular mimicry was one of the mechanisms responsible for immune response against pancreatic b cells in patients infected by E16 and E30 during the 2000 and 2001 meningoencephalitis epidemics in Cuba.
Nevertheless, involvement of other mechanisms in generating a pancreatic autoimmune response cannot be ruled out, since evidence previously discussed suggests that enteroviruses may also cause cytolytic damage to pancreatic b cells. Recent studies in Cuba (unpublished data) have demonstrated replication of epidemic enteroviruses in pancreatic b cells causing cytolytic damage, decrease of insulin secretion and induction of innate proinflammatory immune activation, thus predisposing pancreatic b cells to autoimmune attack.
ENTEROVIRUSES: CAUSAL AGENTS OF TYPE 1 DIABETES?
Based on the evidence presented, we suggest that enteroviruses participate both in preclinical stages of autoimmunity against pancreatic b cells and in the clinical course of diabetes, even in a population exhibiting low T1DM incidence. However, a causal relationship of enteroviruses with T1DM has been difficult to demonstrate due to various obstacles. In most cases, the subclinical stage of the disease may last months and even years; thus, when diabetes is diagnosed, exposure to its triggering agent may have disappeared (hit and run effect).[24] Therefore, any viral effect could have taken place several months or years before T1DM diagnosis, making virus–host interaction difficult to demonstrate.
Another factor creating controversy regarding a possible causal relationship of enteroviruses and T1DM is that enterovirus infections are very common and cause a wide range of diseases, mainly in childhood and adolescence, while T1DM is comparatively less frequent, making it obvious that not all enterovirus infections are associated with progression to T1DM. On the other hand, if enteroviruses constitute environmental factors related to T1DM development, its incidence could be expected to be higher in populations such as Cuba’s, with high prevalence of circulating enterovirus.[8] However, T1DM incidence is highest in European countries such as Finland, Sweden, Germany, Netherlands and England, where prevalence of circulating enterovirus is low.[6,20]
Differences in population genetic composition have been postulated as a possible explanation for this paradox. In European countries, although enterovirus circulation is low, presumably there is a favorable genetic scenario for disease development, due to a higher prevalence of HLA-DR and DQ genotypes, which confer high T1DM risk on the general population. In fact, Ronningen gathered evidence demonstrating a correlation between T1DM incidence in children <15 years of age in Europe and presence of DRB1*0301-DQB1*0201 and DRB1*0401-DQB1*0302 haplotypes, conferring high T1DM risk. These high-risk genotypes are not represented in most populations of Asia and Central America, which could explain the large differences in T1DM incidence in these regions compared to Europe.[25]
Nevertheless, it is interesting that Cuban and European populations share the same susceptibility (HLA DR/DQ) and protection (DRB1*1501–DQA1*0102–DQB1*0602) haplotypes for diabetes development. This is not surprising, because of the contribution of Spanish descent to the genetic structure of the Cuban population. In fact, the Cuban population is genetically closer to Mediterranean and European populations than it is to native American and Asian populations.[26] However, the high degree of intermarriage in Cuba of populations from different geographical origins, quite different from the relative homogeneity of populations in Sweden, Finland and other Northern European countries, could have a protective effect against T1DM development. As it happens, recent studies in Cuba found individuals with a high proportion of European ancestral genes in their genomes more susceptible to developing T1DM than those with a high proportion of African ancestral genes.[27]
Another plausible explanation may be found in the so-called hygiene hypothesis, which postulates that exposure to infectious agents and living in a less hygienic environment ‘educate’ the individual’s immune system, conferring protection against development of allergic reactions. This hypothesis was rapidly extended to other chronic inflammatory and autoimmune processes, such as T1DM.[28] The hypothesis is supported by previous research on poliomyelitis, a disease caused by poliovirus, a member of the enterovirus genus. Risk of damage to motor neurons increases as frequency of poliovirus infection decreases in the population.[29] A similar mechanism may be operative for diabetogenic enterovirus strains: that is, an environment with low frequency of enterovirus exposure limits development of protective immunity early in life for a large number of individuals, leaving them more susceptible to enterovirus infection with the potential to damage pancreatic β cells and initiate autoimmunity.
The hygiene hypothesis is supported by results of various studies in experimental models showing that incidence of autoimmune diabetes can be substantially reduced in inbred non-obese diabetic mice by exposing them to high doses of microbial antigens in early life.[30] The importance of age at exposure for determining whether tolerance or disease develops must be emphasized: mice exposed to microbial agents before the age of five weeks do not develop autoimmune diabetes, but if exposure happens later than that, they do not develop tolerance but rather become diabetic.[30]
Based on these observations and a palpable increase in hygiene in industrialized countries over the past decades, we hypothesize that better hygiene and reduced postnatal exposure to microbial antigens may contribute to increased T1DM incidence in some European countries.
Furthermore, it is accepted that alterations initiated by environmental factors can modulate immune regulation and contribute to an imbalance between protective and pathogenic immune responses.[31] Thus, although our findings are limited to the influence of enterovirus exposure on humoral markers of pancreatic autoimmunity, it is possible that the higher probability of exposure to the agent, due to its high prevalence in Cuba, educates the immune system early in life, favoring development of a protective immune response that predominates over the pathogenic response against pancreatic antigens. It is reasonable to suppose that, while autoimmunity against pancreatic ß cells can be triggered by exposure to diabetogenic enteroviruses, as demonstrated by studies in Cuba, regulatory mechanisms could restrain islet destruction and ultimately abort the autoimmune process.
CONCLUSIONS
Even though research to date has not unequivocally established a direct causal effect of enteroviruses on T1DM, arguments for enteroviruses having a role in its pathogenesis should not be ignored. The evidence presented here reinforces findings in support of the hypothesis that enteroviruses act as risk factors for diabetes. Molecular mimicry and acute cytolytic damage are cited as mechanisms by which enteroviruses can mediate an immune response directed against pancreatic b cells.
Since there is no cure for the increasing number of patients with T1DM, it is of utmost importance to develop ways to prevent autoreactivity or reintroduce immune regulation after autoimmunity is established. If progress thus far motivates the scientific community to investigate possible T1DM viral causes and evaluate enteroviruses’ ability to generate protective immune mechanisms in humans, it is not unreasonable to think that in future this chronic non-communicable autoimmune disease could be prevented by vaccines against human enteroviruses.





