INTRODUCTION
Globally, diseases due to chromosomal abnormalities are relatively frequent in human populations. Their incidence is 6 per 1000 live births, and the most common of these is Down syndrome, occurring in 1/800 live births.[1–3] Antenatal cytogenetic testing (ACT) is used to assess the fetal chromosome complement; it detects constitutional chromosomal abnormalities that can cause serious problems for an affected child, impacting the child’s and family’s quality of life. From a social perspective, such testing has three benefits: by detecting chromosomal diseases, the child’s care can be planned in advance, leading in many cases to greater possibilities for survival and better quality of life; it provides families the choice of interrupting pregnancy where this is legally permissible; and offers reassurance to the majority of couples who have normal results.
Multiple factors are involved in ACT, including genetic counseling; sample collection by the obstetrician; sample transportation; conditions for culture and processing; and chromosome analysis, in the end determining the fetal karyotype.[4]
The various ACT methods are classified by gestational age when they should be performed and type of tissue sampled for testing. Chorionic villus sampling (CVS) is done in the first trimester of pregnancy and results are ready in less than a week, since cell culture is not necessary.[5] Amniocentesis is usually performed during the second trimester, the amniotic fluid cultured to procure cell colonies to obtain fetal chromosomes.[4] Cordocentesis, collection of fetal blood, is generally performed in the third trimester and before 26 weeks of pregnancy. It is not recommended as a routine test because of increased risk of spontaneous abortion and is only justified when amniocentesis results are inconclusive or ultrasound (US) findings suggest possible fetal malformation. Fetal blood is cultured and the results should be obtained in less than a week.[6] The technique of fluorescence in situ hybridization (FISH) applied to amniotic interphase cells can be performed from 10 weeks until third trimester; it does not require cell culture and the result is obtained in two days. It is used for diagnosis of Down syndrome and other relatively frequent chromosomal abnormalities, but is not useful for diagnosing structural chromosome abnormalities.[7]
Collaborative studies have been carried out in high-income countries experienced with ACT.[8–11] However for many reasons (such as health policies, religious beliefs, and illegality of abortion even in presence of a severe genetic anomaly in the fetus), most Latin American and Caribbean countries have not implemented ACT in a way that provides general access to their populations.[12–16] In addition, in most developing countries, such diagnostic programs for genetic diseases are a luxury due to their high cost.
In Cuba, pregnancies with potential risk for Down syndrome and other chromosomal disorders are identified at the primary care level by family physicians and evaluated by municipal clinical geneticists and in provincial genetics centers. In Havana, high-risk pregnant women are referred to the Provincial Medical Genetics Center, where they receive genetic counseling and undergo US before sampling. In all cases, genetic counseling is voluntary and informed consent is required from couples before carrying out any invasive procedure. ACT is free, as are all medical genetics services for pregnant women at all levels of the health system, through tertiary care.[17] Figure 1 provides a summary of the case management algorithm followed with pregnant women in Cuba and how they are enlisted into the genetics program.
Figure 1: Algorithm for care of pregnant women with genetic risk
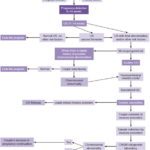
CNGM: National Medical Genetics Center CPGM: Provincial Medical Genetics Center US: ultrasound
These services are made possible under the National Program for Diagnosis, Management and Prevention of Birth Defects and Hereditary Diseases, begun in Cuba in Havana City Province in the early 1980s. Specifically, the cytogenetic laboratory of the National Medical Genetics Center (CNGM, the Spanish acronym) was established in 1984, at first serving patients in Havana, and later Cuba’s western provinces.[17] Other cytogenetic laboratories were subsequently created in most Cuban provinces: Pinar del Río, Matanzas, Villa Clara, Sancti Spíritus, Ciego de Ávila, Camagüey, Granma, Las Tunas, Holguín, Santiago de Cuba and Guantánamo, but ACT methods such as cordocentesis and FISH in amniocytes are only performed in CNGM’s laboratory, where samples are received from throughout Cuba. There has been no comprehensive study of the work of CNGM’s cytogenetics laboratory over its history. Hence the objective of this study was to describe ACT results from 1984 through 2012 by diagnostic method and testing indication.
METHODS
Type of study, universe and sample A descriptive retrospective study was carried out, based on administrative data for all antenatal diagnoses during the 1984–2012 period. The universe consisted of 24,074 pregnant women registered in the cytogenetic laboratory of the National Medical Genetics Center, Havana. Their data were reviewed and cases with a conclusive ACT result (≥10 metaphases analyzed and 2 karyotypes examined) were selected. In some cases, a second type of ACT was needed to corroborate an inconclusive first test. ACT of monozygotic twins was excluded from the study because diagnostic certainty about two fetuses is impossible with a single sample. ACT was performed independently for each fetus in the case of dizygotic twins, considering them separate samples. A total of 22,928 women constituted the final study sample.
Table 1: Study variables

ACT: antenatal cytogenetic testing
Data acquisition Four cytogenetic laboratory databases for 1984–2012 were reviewed, those for analysis of fetal cells in amniotic fluid, chromosome complement study by CVS, cordocentesis (studying fetal cells in umbilical cord blood), and FISH using amniotic fluid fetal cells (amniocytes). For each method, data were collected on number of tests performed, testing indication, number and type of chromosomal abnormalities detected, and decision regarding pregnancy continuation after abnormal ACT results.
Study variables are described in Table 1.
ACT methods used in the CNGM laboratory Various technologies and culture media have been used over the 28 years covered by this study, and working conditions have been gradually modernized.[18] The technology and culture media used in most recent years are described here. For chromosomal diagnosis by any ACT method, the software Cytovision version 3.9, section Genus (Applied Imaging, USA) is used for image capture, processing and analysis. Images are obtained by bright-field and fluorescence microscopy (Olympus BX51, Japan).
Chorionic villus sampling This is performed between 10 and 14 weeks of gestation. US-guided sample aspiration is done by percutaneous transabdominal technique or the transvaginal/transcervical approach to collect a sample of the chorion frondosum, tissue of the same embryological origin as the fetus (indirect diagnostic method, because fetal tissue per se is not sampled). The sample is processed within 48 hours of arrival at the laboratory. The culture media used is RPMI 1640 Medium (Irvine Scientific, USA) or Amcell Grow (Labs-Biotechnology, Germany). Obtaining chromosomes is very fast. Slides are examined after enzymatic treatment with trypsin (1:250) and staining for adequate definition of chromosome structure. Diagnosis is obtained two to five days after sampling.
Amniotic fluid culture This is carried out between 16 and 20 weeks of gestation. By US-guided transabdominal puncture (amniocentesis), 20 mL of amniotic fluid are collected, containing fetal cells from the skin, genitourinary and gastrointestinal tracts. Amniocytes (cells of choice for ACT), are cultured in a sterile medium (complete AmnioMax II, Gibco, USA) at 37 oC; when they reach optimal growth, cell colonies adhering to the surface of the culture flask are harvested to obtain fetal chromosomes. Diagnosis is performed on slides, the chromosome preparation first treated with trypsin (1:250) and Giemsa stain to achieve proper visualization. Final results are obtained 12–15 days after amniocentesis. Amniotic fluid without centrifugation is generally used for amniocyte culture. Four flasks are seeded per patient and the remaining amniotic fluid, properly identified, is stored at 4 oC for 20 days, for use in case of problems with the first culture (to avoid subjecting the pregnant woman to a second amniocentesis).
Cordocentesis This is done between 21 and 25 weeks by transabdominal US-guided puncture to find the umbilical cord insertion on the maternal surface of the placenta and collect fetal blood. The sample is cultured in sterile medium (Quantum-PBL, USA). The process for obtaining chromosomes is carried out in 72 hours; slides are treated with trypsin (1:250) and stained with 5% Giemsa to diagnose fetal chromosomes. The result is obtained in three to four days.
FISH in interphase amniotic cells This test was standardized in the laboratory in 2010 and is performed from 10–31 weeks’ gestation. Amniotic fluid fetal cells are obtained by amniocentesis. The sample is not cultured, but processed directly, applying two enzymatic treatments to the amniocytes: first trypsin (1:250) and then pepsin (10 mg/mL). The preparation is smeared on slides and hybridized with probes identifying chromosomes 21, 18, 13, X and Y. To identify chromosomes 18, X and Y, Aneuvision probe kits (Vysis, USA) are used for α-satellite CEP 18 probes (p11.1-q11.1) labeled with fluorochrome SpectrumAqua, X (p11.1-q11.1) labeled with SpectrumGreen fluorochrome and Y (p11.1-q11.1) labeled with SpectrumOrange fluorochrome. To identify chromosomes 13 and 21, Aneuvision probe kits (Vysis, USA), LSI 13 (13q14) probes labeled with SpectrumGreen fluorochrome and 21 (q22.13-q22.2) probes labeled with SpectrumOrange fluorochrome. On the next day they are washed to remove excess label; diamidino phenylindole (DAPI) stain is applied and they are analyzed by fluorescence microscopy. The result is obtained in two days.[19]
Analysis of chromosomal mosaicism in ACT Chorionic villus sampling When two cell lines appear, the finding is corroborated by amniocyte culture.[18]
Amniotic fluid Cases of chromosomal mosaicism in amniocyte culture are considered positive when the same chromosomal abnormality is detected in at least two different primary culture flasks, after application of Hsu and Benn guidelines.[20]
Cord blood Mosaicism is diagnosed when the same chromosomal anomaly appears at least twice in 30 metaphases analyzed.[6]
FISH in interphase amniotic cells Mosaicism is diagnosed when the percentage of abnormal cells exceeds 10% of the total 200 cells analyzed and there are also anomalies in the fetal US or suspicion of mosaicism from a previous amniocentesis.[21]
Data analysis For chromosomal anomaly analysis (mosaicism or pure lines) the following were considered:
- the most frequent autosomal aneuploidies, trisomy 21 or Down syndrome, trisomy 18 or Edward syndrome and trisomy 13 or Patau syndrome
- the most frequent sex chromosome aneuploidies, such as Turner syndrome (45,X); 47,XXX; Klinefelter syndrome (47,XXY) and 47,XYY
- apparently balanced structural alterations, such as translocations, inversions and insertions
- unbalanced structural alterations, such as deletions, duplications, isochromosomes and rings
- marker chromosomes, rare aneuploidies (of chromosomes 22 and 9), triploidies, X-chromosome polysomies and 46,XY/46,XX, as included in the other chromosome abnormalities category
For each variable, absolute numbers were obtained and percentages calculated to compare results.
Ethical considerations In the program, genetic counseling is provided to the couple and informed consent is obtained for any invasive sampling procedure. Once testing is complete, all unused samples are discarded. Patient identification codes in laboratory databases were used for this study, data analysis procedures preserving patient anonymity. CNGM’s ethics committee approved the study.
RESULTS
Table 2 summarizes the results of reasons for indicating antenatal studies by different ACT methods. Advanced maternal age (AMA) was the main testing indication (69.2%), as it was the major one (70%) for the two most frequent methods. Parental carriage of chromosomal rearrangement was the least common testing indication (0.4%). AMA was also the most frequent indication for cordocentesis (45%), but there was also a large proportion of reasons grouped as “other” (30.5%). Diagnostic corroboration of inclusive first-test results weighed especially in the other category. Suspicion of chromosomal abnormality from fetal US findings was the main indication for FISH (55.6%).
Table 2: Indications for ACT by method
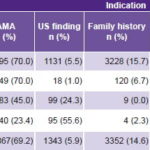
ACT: antenatal cytogenetic testing AMA: advanced maternal age
FISH: fluorescence in situ hybridization US: ultrasound
Other: chromosomal anomalies in previous pregnancies, sex determination in X-linked recessive diseases, exposure to mutagenic agents, maternal anxiety
The positivity rate in pregnant women studied was 2.8% (641/22,928), taking into account all ACT variants used. Table 3 reveals that the highest positivity rates appeared in the methods least frequently used. FISH, which only accounted for 0.7 of tests (171/22,928), contributed 2.7% of positive cases (17/641).
For those invasive procedures in which AMA was the most common ACT indication (amniocyte culture and CVS), the positivity rate was <3%. In cordocentesis and FISH, in which other indications predominated, anomaly detection rates were 6.9% and 9.9%, respectively.
Table 3: Positivity rates by ACT method
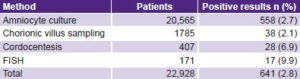
ACT: antenatal cytogenetic testing / FISH: fluorescence in situ hybridization
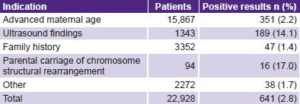
When AMA was the testing indication, the positivity rate was only 2.2% (351/15,867). The highest positivity rates were detected in association with parental carriage of balanced structural chromosome abnormalities (17%) and with suspicious US findings (14.1%)(Table 4).
Table 4: Positivity rates by testing indication
Of the 641 positive cases, 569 had pure lines (88.8%) and 72 chromosomal mosaicisms (11.2%). Down syndrome predominated, with 291 cases (279 pure line and 12 mosaic trisomy,
totaling 45.4% of cases); followed by Edward syndrome with 77 cases (71 pure and 6 mosaic; 12% of cases); and Patau syndrome, 30 cases (27 pure and 3 mosaic, 4.7% of cases).
The predominant sexual aneuploidies were 45,X and 47,XXX, both in pure lines and mosaics. In the category of structural chromosome abnormalities, the majority, 102 patients (15.9%), were apparently balanced and only 42 (6.6%) were unbalanced (Table 5).
In the other chromosomal abnormalities category, one patient was diagnosed with mosaic of chromosome 9 trisomy; 3 with mosaic trisomy 22; 1 with mosaic trisomy 8; 1 with triploidy (69,XXY) and two samples showed chimerism 46,XY/46,XX, all very rare events.
Supernumerary marker chromosomes were also included in this category (pure lines and mosaics) with de novo markers (7 cases) predominating over inherited ones (3 cases); in two patients, determination of the marker chromosome’s origin was impossible because study of both parents was not feasible. Four cases were diagnosed with trisomy 20 mosaics.
ACT by amniotic fluid culture found 56 cases of chromosomal mosaicism (0.27% of tests done, 56/20,565). Five cases of chromosomal mosaicism were found through studies of cord blood (5/407, 1.2%) and 3 cases by FISH (3/171, 1.7%), where an unusual case of X-chromosome polysomy was diagnosed: 49,XXXXY/48,XXXY.
Eight cases of chromosomal mosaicism were detected by CVS, most corroborated by analysis of amniotic fluid cells. Sex chromosome mosaics were the most frequent, 43% (31/72); followed by autosomal, 36.1% (26/72); those of marker chromosomes, 13.9% (10/72); and structural abnormalities, 8.3% (6/72). The chromosomes most frequently involved in cases of chromosomal mosaicism were X, 21, 22, 18, 13 and 20.
Most parents chose to continue the pregnancy when apparently balanced structural chromosomal abnormalities were found (96 cases of pure lines and 2 cases of mosaicism), except in 4 cases of de novo abnormalities, in which parents opted for termination (Table 5).
Conversely, in most cases of unbalanced structural abnormality, couples chose to terminate pregnancies, except in three cases: one with a deletion of the short arm of the X chromosome, one with an addition to the short arm of chromosome 12, and a third with an addition to the short arm of chromosome 22 (Table 5).
In total, 78.6% (504/641) couples decided to interrupt pregnancies. In cases of autosomal aneuploidy, 97.5% (388/398) of couples chose pregnancy termination; except for 9 with Down syndrome and 1 with trisomy 13. For sexual aneuploidies, 21 of 74 couples (28.4%) continued pregnancy: 12 with 47,XXX; 4 with 45,X; 3 with 47,XXY and 2 with 47,XYY (Table 5).
Table 5: Chromosomal abnormalities in pure lines and mosaics detected in ACT, and couple’s decisions regarding pregnancy

ACT: antenatal cytogenetic testing AF: amniotic fluid CB: cord blood
CVS: chorionic villus sampling FISH: Fluorescence in situ hybridization
SA: structural abnormalities
a There was a mosaic involving structural error of sex chromosomes.
b Includes 5 autosomic mosaics (3 of chromosome 22, 1 of 9 and 1 of 8).
DISCUSSION
For more than 30 years, ACT has been used worldwide to determine the fetal chromosome complement.[22] Since 1984, the CNGM cytogenetic laboratory in Havana has provided these services, mainly for western Cuba.
In Cuba, AMA is the main indication for ACT, as reflected in this study. After 35 years of age, the mother’s risk of having a child with a chromosomal disorder increases because of errors in maternal meiosis.[23] Because Cuba does not have the financial resources for testing all pregnant women aged ≥35 years, ACT begins at age 37 years.
The ACT results we observed are similar to those of other authors. Nagel in the Netherlands (10 years of data),[11] Kessler in Brazil (905 samples),[12] and Comas in Spain (10 years of data)[24] also reported that AMA was the most frequent basis for test indication, with a positivity rate of 2%–3%. Generally, when AMA is the most frequent indication, numerical abnormalities, Down syndrome among them, predominate.[9–12,24]
Positivity rises considerably when the fetus has US-detected malformations (cardiovascular, renal system, anterior ventral wall and other malformations).[25–28] As early as 1985, Benacerraf reported a strong association between fetal nuchal fold and trisomy 21.[27] Currently, other US markers (nuchal translucency, nasal bone length, femoral or humeral length, intracardiac echogenic focus, echogenic bowel and pyelectasia) are used to determine risk of the most frequent aneuploidies (trisomies 21, 18 and 13).[28] So it is not surprising that US findings constituted the testing indication with the second highest positivity rate in our study.
As expected, the highest percentage of positive cases was found when testing indication was parental carriage of a balanced structural rearrangement. These parents have a high probability of transmitting chromosomal rearrangements to their offspring, whether such rearrangements are balanced (alternate segregation) or unbalanced (mainly adjacent segregation).[29]
Family history of chromosomal abnormalities (mainly aneuploidies) is a very weak risk predictor of chromosomal abnormalities during ACT (about 1% risk). The procedure is carried out mainly to relieve parental anxiety about their baby’s health.[29]
The other category includes cases referred because of abnormal biochemical markers in maternal serum, for sex determination in X-linked recessive diseases, and to assuage parental anxiety. Of these, abnormal biochemical markers may contribute most effectively to antenatal detection of chromosomal abnormalities. Several authors affirm the effectiveness of biochemical markers in detecting increased risk of chromosomal aneuploidy,[30–32] but unfortunately, in Havana this testing was only done in 2002, coinciding with an increase of Down syndrome diagnosed antenatally in that year (24 cases, compared to only 16 in 2001, among approximately the same number of tests).
Sex determination in X-linked recessive diseases is performed because boys have a 50% risk of developing the disease. In the case of metabolic diseases (which are monogenic), there is no increased risk of chromosomal abnormalities and ACT is performed to achieve good cell proliferation for enzymatic quantification to determine fetal health. Maternal anxiety is an indication when, with no objective genetic risk, the pregnant woman insists upon this study. All this explains the low proportion of positive cases detected in the other category of ACT indications.
Only three well-defined chromosomal disorders in pure lines that are trisomies of an entire autosomal chromosome are compatible with postnatal survival: trisomy 21 (Down syndrome), trisomy 18 (Edward syndrome) and trisomy 13 (Patau syndrome). Fetuses affected by pure trisomies of the remaining autosomes are aborted early, and the zygote may not even be implanted,[23] which prevents detection during ACT. Down syndrome is the most common and best known chromosomal disorder and the main genetic cause of mental retardation.[23]
Couples who choose to continue pregnancy after being informed of their child’s chromosome abnormality may do so for various reasons, including religious beliefs, a strong desire for a child, twin pregnancy in which one fetus is normal (in Cuba selective abortion of the affected fetus was not possible during the study period) and history of infertility in the couple.
In the category other chromosomal disorders, we reported a case of triploidy, an abnormality that occurs in 2% of conceptions, of which only 3% of infants survive.[33] Other infrequent cases reported in this study are mosaic trisomies 9 and 22. In his 1997 research, Hsu managed to collect 277 cases of rare autosomal aneuploidy mosaics (excluding the most frequent, of chromosomes 21, 13 and 18) and found only 25 cases of mosaic trisomy 9 and 11 of mosaic trisomy 22.[34] Chromosomal mosaicism is detected in 0.1%–0.3% of ACT.[35] A rare case of X-polysomy with 49,XXXXY/48,XXXY was also diagnosed, an event that occurs in 1/10,000 boys.[33] Mosaic trisomy 20 (we observed 4 cases) is usually associated with placenta-confined mosaicism and does not pose a threat to fetal health; there is no established chromosomal syndrome for this type of mosaicism.[36]
Amniocyte culture predominated among ACT techniques because it is considered a highly reliable diagnostic method, with very low levels of fetal loss after amniocentesis.[4,16]
Diagnosis by CVS has not gained the acceptance originally expected by cytogeneticists, among other reasons because it causes a higher rate of fetal loss, there is higher incidence of false positives and negatives (due to placenta-confined mosaicism), the quality of the chromosomes obtained is inferior to that of chromosomes obtained by amniocentesis, and another invasive procedure is sometimes needed (generally amniocentesis) to corroborate diagnosis.[5]
With the recent introduction of FISH, basically for high-risk cases (suspicion of fetal abnormalities by US), the percentage of chromosomal abnormalities detected increased substantially. This has led to reduction in the number of cordocenteses performed. During 2009–2011, an average of 73 cordocentesis were performed annually; in 2012, there were only 29. Cordocentesis is an invasive test that also carries with it greater risk of abortion and technical difficulty, which also influenced this reduction.
This study found a high frequency of interruptions of pregnancies when ACT detected genetic risk in the offspring. This bears out consensus among Cubans concerning abortion as an acceptable reproductive choice when there is risk of genetic disorder. In cases of a genetic disorder or other condition that poses a serious threat to the survival or quality of life of the fetus, therapeutic abortion is legal in Cuba up to 26 weeks of pregnancy, always with approval by a medical panel.[37] However, the couple’s decision is respected if they decide to continue despite positive diagnosis of a chromosomal abnormality. This is in compliance with WHO’s ethical guidelines for medical genetics, which state that ACT should only be carried out for reasons relevant to fetal health and should be offered regardless of the couple’s beliefs about abortion.[38]
One limitation of this study was the impossibility of confirming cytogenetic diagnosis in abortive material of some cases, especially those with unusual diagnoses (e.g., mosaic trisomy 9, 22 and 8; and 46,XY/46,XX). This was because pregnancy termination took place in hospitals far from the laboratory, making it difficult to collect tissue samples, or because the parents refused consent, mainly for emotional or religious reasons. Another limitation is that the analysis did not take into account patient residence; most were from Havana, but until 2004 many cases were studied from throughout western Cuba. It would be useful to consider this variable for future studies so that results, in addition to presenting the overall work of the laboratory, may be associated more clearly with patients’ place of residence.
We believe that the main benefit of an antenatal detection program for genetic disorders is social, since parents are provided information about the genetic health of their offsprings and, based on reliable diagnoses, can decide whether to continue the pregnancy. This can help prevent suffering in families where children could be born with chromosomal disorders or, in the case of continuing with the pregnancy, help them prepare psychologically for raising a child with physical and intellectual disabilities. Another benefit of ACT is to provide most couples the certainty of normal chromosomal results.
The March of Dimes Global Report on Birth Defects[39] lists Cuba as one of the countries with lowest prevalence of Down syndrome (0.7/1000 live births). Cuba’s ACT program, universal access to health services and the National Network of Medical Genetics have contributed to this status.[40] At the same time, Down syndrome children and adults receive specialized care in the public health system and 396 special schools throughout Cuba.[41]
Despite achievements reported in this paper, better strategies are needed to increase chromosomal abnormality detection rates in ACT, in order to further reduce genetic disorders in the Cuban population. When AMA is the main indication, chromosomal abnormality detection is low and a large proportion of the maternal population (aged < 37 years) do not participate in the program. The combination of biochemical markers in maternal serum and findings of fetal anomalies detected by US during pregnancy is useful in estimating increased risk of chromosomal abnormalities, regardless of maternal age,[30,31] as was shown by its application in Havana in 2002.[40] This would make it feasible to reduce invasive procedures during pregnancy and to considerably increase detection of the most frequent chromosomal abnormalities.
CONCLUSIONS
Antenatal cytogenetic testing has helped reduce chromosomal abnormalities, mainly in Havana, and has provided reassurance of chromosomally normal children for couples at high genetic risk. The percentage of continuing pregnancies after a diagnosis of major chromosomal abnormality has been low, supporting evidence of broad population acceptance of abortion as an option when severe genetic abnormalities are present.
ACKNOWLEDGMENTS
The authors thank Havana’s geneticists, genetic counselors and cytogeneticists for their contributions to this study.









