INTRODUCTION
Erythropoietin (EPO), a glycoprotein manufactured mainly in the kidney, is the main factor regulating production of red blood cells and is essential for their proliferation, differentiation and maturation in bone marrow.[1,2] Discovery of the human EPO gene in 1985 enabled development and production of recombinant erythropoietin (RHuEPO) for clinical use, transforming anemia treatment, particularly for patients with end-stage renal disease.[3–5] Several clinical studies of dialysis patients receiving RHuEPO showed erythropoiesis stimulation and correction of renal anemia, fewer transfusions and, as a result, improved quality of life.[6–8]
RHuEPO products, including epoetin alpha (Eprex® Epogen®, Procrit®), epoetin beta (NeoRecormon®), darbepoetin alpha (Aranesp) and CERA (Continuous Erythropoietin Receptor Activator) are currently classified and marketed internationally as erythropoiesis stimulating agents (ESA).[9] ESAs have been used effectively to treat anemia associated with a variety of conditions, most frequently chronic kidney disease (CKD), prematurity, and HIV infection. They are also used to increase Hb levels during surgery; following radiotherapy, chemotherapy or transplant; in situations when red blood cell transfusion is ruled out; or to improve autologous transfusion response.[10]
In Cuba, however, patients with anemia due to CKD were unable to benefit from RHuEPO treatment due to the high price of ESAs in the international market. The Molecular Immunology Center (CIM, its acronym in Spanish) therefore began RHuEPO research and development, obtaining a product registered as ior®EPOCIM in 1998 by the national drug regulatory agency CECMED (its acronym in Spanish).[11] Today, ior®EPOCIM is widely used in patients with anemia due to CKD, at a price the Cuban public health system can afford. All treatment is provided at no cost to patients.
Use of ior®EPOCIM in dialysis patients in Cuba has shown beneficial effects, such as correction of anemia, reduced need for transfusions, higher quality of life, increased capacity for work, and improved cognitive functioning. The product has shown good safety parameters; no severe adverse events associated with its administration have been reported.[12,13]
Malignant diseases are frequently accompanied by anemia, which may be aggravated by cancer treatment or other causes, including malnutrition, hemorrhage, and tumor-cell invasion of bone marrow. Anemia, in turn, may negatively affect quality of life due to accompanying asthenia, fatigue, and other symptoms.[14–16]
RHuEPO administration and red blood cell transfusions are the most widely used treatments for chemotherapy-induced anemia in cancer patients with Hb <100 g/l.[17,18] Studies of RHuEPO administration in adult and pediatric cancer patients have shown correction of anemia, along with reduced need for transfusions and their associated risks, all of which contribute to improving quality of life.[19–21] The few studies of RHuEPO use in pediatric cancer patients with anemia report a 20 g/l Hb increase in 46–75% of patients, as well as a considerable reduction in transfusion requirements.[21–24]
American Association of Clinical Oncology (ASCO) guidelines recommend RHuEPO treatment for cancer patients with chemotherapy-induced anemia and Hb ≤100 g/l, without differentiating between adults and children.[17] The French Cancer Institute’s Recommendations for Erythropoiesis Stimulating Agents in the Management of Anemia in Children with Cancer indicate that the decision to treat with ESAs should be made case by case, and caution against systematic administration of ESAs for a prolonged period, while noting there does not seem to be association between ESA use as indicated and significant toxicity in children.[18]
In Cuba in 2008, a Phase IV clinical trial was conducted to assess the efficacy and safety of ior®EPOCIM treatment for anemia in 338 adult patients with radiotherapy- or chemotherapy-induced anemia. Patients received 10,000 U ior®EPOCIM subcutaneously, 3 times a week for 8 weeks. Results showed a 21.7 g/l increase in mean Hb level and a 31.4% reduction in transfusions at conclusion of the study. The most frequent adverse events were pain at the injection site and bone pain: 23.5% and 13.8%, respectively, of total events reported.[25]
Considering the results of that clinical trial, as well as a literature review of studies treating pediatric patients with RHuEPO, and the current international guidelines mentioned above, a clinical trial was conducted to assess the efficacy and safety of ior®EPOCIM in pediatric cancer patients with radiotherapy- or chemotherapy-induced anemia. The working hypothesis posed an Hb increase ≥15 g/l in 70% of patients receiving ior®EPOCIM for 8 weeks.
METHODS
Study Design An open Phase IV non-controlled, multicenter clinical trial was conducted. The study universe was comprised of 157 pediatric cancer patients who fulfilled inclusion criteria and were treated between September 2004 and August 2006 in the oncology or hematology departments of 8 participating hospitals in Cuba.
Inclusion criteria Patients aged 1–19 years with cyto-histological diagnosis of cancer in any location and anemia clinically associated with chemotherapy or radiotherapy, not previously treated with RHuEPO. Anemia was defined as Hb <110 g/l for patients aged 1–11 years and Hb <120 g/l for patients aged 12–19 years.[26]
Prior to inclusion in the study, written informed consent was obtained from a parent or guardian of each patient, signed in the presence of the clinical investigator in the participating institution and a witness.
Exclusion criteria Known hypersensitivity to products derived from higher cells or human albumin, active hemorrhage or hemolysis, or uncontrolled high blood pressure at time of inclusion.
Suspension criteria Hb ≥140–150 g/l achieved prior to week 8 of treatment; appearance of an exclusion criterion; adverse event implying risk to the patient, at the discretion of the clinical investigator; patient’s death.
Ethical Considerations The research protocol was approved by the Clinical Research Ethics Committees of the participating institutions. The national drug regulatory agency, CECMED, was notified as required for Phase IV clinical trials of a registered product. Helsinki Declaration criteria,[27] International Ethical Guidelines for Biomedical Research involving Human Subjects,[28] and Standard Working Procedures of the National Coordinating Center of Clinical Trials (CENCEC, its acronym in Spanish)[29] were followed.
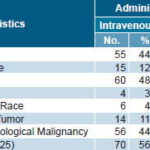
Treatment Patients received either 600 U/kg ior®EPOCIM intravenously, once a week, or 150 U/kg ior®EPOCIM subcutaneously in the right deltoid muscle, 3 times a week, for 8 weeks, consistent with International Guidelines for the use of Erythropoiesis Stimulating Agents.[17] Administration route was assigned by the clinical investigator, according to the patient’s chemotherapy schedule. The intravenous route was used in 81 patients, and the subcutaneous route in 76 patients. Dosage for each patient was calculated by the clinical investigator, based on patient’s weight and administration route. Patients were hospitalized or ambulatory during treatment, at the discretion of the clinical investigator, taking into account each patient’s clinical status.
Hb levels were measured weekly, as well as hematocrit, and reticulocyte and platelet counts. Patients whose Hb dropped below initial levels were given transfusions, at the discretion of the clinical investigator, and continued ior®EPOCIM treatment.
Hb levels were evaluated at the conclusion of week 4 of treatment, and the ior®EPOCIM dose was maintained in patients whose Hb had risen ≥10 g/l above initial values. In patients whose Hb had not increased ≥10 g/l above initial values, the intravenous dose was increased to 900 U/kg weekly, and the subcutaneous dose to 300 U/kg three times a week.
Data collection and analysis A data collection logbook was kept for each patient, completed by the clinical investigator in each participating institution. The patient’s clinical evolution was recorded weekly during the 8-week study period, as well as weight, ior®EPOCIM dose, and lab test results (hemoglobin, hematocrit, reticulocyte and platelet count at treatment onset and termination, serum iron levels and transferrin saturation index).
Efficacy was measured against the study hypothesis and transfusions required during the study compared to the number of transfusions in the 8 weeks prior to inclusion in the study.
Hb response during ior®EPOCIM treatment was evaluated by calculating the difference between initial and final mean Hb levels.
Safety was measured by registry and analysis of adverse events (AE), defined as any harmful clinical event in a patient receiving the product under study, not necessarily implying a causal relationship with the treatment. AEs were recorded cumulatively by the clinical investigator at the conclusion of week 4 and week 8.
Adverse events were classified by intensity and causality according to CENCEC’s Standard Working Procedure for registering adverse events.[29] The Jones algorithm was also used to classify causality.[30] Intensity was classified as mild, moderate, less severe, or severe; and causality was classified very probable, probable, possible, or remote. In accordance with CENCEC’s procedures, only those AE classified Very Probable were considered associated with use of the medication.[29]
Data analysis was performed by “protocol” (only data from patients completing treatment) and by “intent to treat” (data from all patients initially included in the study). Databases were created in Excel spreadsheets and processed using SPSS version 10.0 statistical software package.
RESULTS
Of 157 patients initially included in the study, 129 completed treatment and 125 were included in the data analysis. After 8 weeks of ior®EPOCIM treatment, 68.8% of patients (86/125) attained an Hb increase ≥15 g/l, and 59.2% (74/125) attained an Hb increase ≥20 g/l (Table 1). In the intent-to-treat analysis, 54.8% of patients (86/157) attained an Hb increase ≥15g/l, and mean Hb rose 20.94 g/l, from 90.14 g/l at study onset to 111.08 g/l at termination.
Table 1: Hemoglobin Increase at Week 8 of ior®EPOCIM Treatment
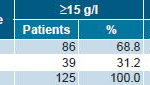
p= 0.41978 (Pearson’s chi-square test)
Treatment was suspended before 8 weeks in 28 patients: 8 withdrew voluntarily; 9 were withdrawn by the clinical investigator because of adverse events; 6 were withdrawn due to irregularities in ior®EPOCIM distribution; and 5 patients died as a result of their disease. Data from 4 patients who completed treatment were not analyzed because final Hb lab results were not available.
The majority of patients completing treatment were male (72.0%) and white (72.8%) (Table 2). Mean age was 6.85 years (age range 1–19 years), and mean weight was 27.2 kg (weight range 9.0–106.0 kg). Mean weight by administration route was 26.0 kg for those receiving ior®EPOCIM intravenously and 28.5 kg for those treated subcutaneously.
Table 2: Patient Characteristics and ior®EPOCIM Administration Routes
Expected response (Hb increase ≥15 g/l) at 8 weeks was significantly greater in patients receiving ior®EPOCIM subcutaneously (78.2%) compared to those treated intravenously (61.4%) (Table 3).
Table 3: Patients with ≥15 g/l Hemoglobin Increase at Week 8 of ior®EPOCIM Treatment, by Administration Route

p= 0.41978 (Pearson’s chi-square test)
During the study, the number of patients requiring transfusions declined 15.3%, and the number of transfusions decreased 17.1%. In the 8 weeks preceding onset of ior®EPOCIM treatment, 91 patients (58.0% of study universe) received a total of 181 transfusions, compared to 67 patients (42.7% of study universe) requiring a total of 150 transfusions during treatment.
In the 157 patients exposed to ior®EPOCIM treatment, 166 adverse events were reported: 94 in weeks 1–4 and 72 in weeks 5–8. The most frequent AE was fever (19.3%), followed by vomiting (10.2%), flu-like syndrome (9.6%), and weight loss (8.4%) (Table 4). Of total AE reported, 45.2% were categorized Other, appearing in only one patient and, when analyzed, were found mainly associated with the patient’s disease.
Table 4: Adverse Events at Week 4 and Week 8 of ior®EPOCIM Treatment*
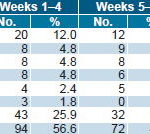
* Intent-to-treat analysis of 157 patients initially included in the study
In the causality analysis only 7 AE (4.2%) were classified as having a very probable association with exposure to ior®EPOCIM, none of these severe; 2 fever events were considered mild; 1 bone pain, 1 redness, and 1 vomiting, all moderate; 1 burning sensation and 1 pain at injection site were classified as less severe. Remaining AE were classified as Probable (5.5%), Possible (65.5%) and Remote (24.8%) (Table 5).
Table 5: Adverse Events by Causality after ior®EPOCIM Treatment*
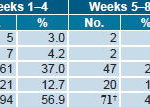
* Intent-to-treat analysis of 157 patients initially included in the study † Information missing for 1 event at Week 8
DISCUSSION
The 68.8% response in the present study was considered clinically acceptable, even though below the 70.0% posed in the working hypothesis. It also falls within the 46%–75% response range for mean Hb increases ± 2 g/dl (20 g/l) at 8 and 12 weeks of treatment, reported in international studies using epoetin alpha to treat pediatric cancer patients with anemia.[20,22–24]
In a study at St. Jude Children’s Research Hospital in the United States, 217 patients aged 5–18 years were randomized to receive 600 U/kg of epoetin alpha intravenously or placebo. On day 29, a 2 g/dl (20 g/l) Hb increase was attained in 56.5% of the epoetin-treated group and 34.9% of the placebo group.[31]
Researchers in Greece reported a 2 g/l Hb increase at 8 weeks in 46% of 50 patients with hematologic malignancies and solid tumors, treated with 150 U/kg, 3 times a week.[24] A higher response was obtained in a study conducted in Spain to evaluate epoetin alpha in 25 patients with different types of solid tumors. They received 150 U/kg of epoetin alpha, 3 times a week, for 3 months and attained a 2 g/dl (20 g/l) Hb increase in 75% of patients.[23]
In a study carried out in Turkey, 34 children aged 1–16 years with solid tumors were randomized to receive 150 U/kg of epoetin alpha or placebo, 3 times a week, for 8 weeks. A significant decrease in the number of patients requiring transfusions and a 20 g/l increase in mean Hb were obtained in the treated group, while there was no change in the control group.[21]
According to these and other published studies, RHuEPO is safe and well-tolerated as used to correct anemia in pediatric cancer patients. None of the most frequent adverse events reported in the present study were mentioned in any of the studies reviewed, and those AE reported were mostly transient, local reactions, such as redness and burning sensation.[21–24,31–33]
Although weight loss was the fourth most frequent AE reported in the present study, it was considered associated with patients’ primary disease and not with treatment, as other authors have explained.[34,35]
In the present study, a better response was achieved with subcutaneous administration of ior®EPOCIM than with intravenous administration. Similar results have been reported in adult hemodialysis patients with anemia,[36,37] but we found no other studies comparing response by administration route of RHuEPO in children or adults with cancer. A limitation of the present study was lack of an established, uniform, criteria for assigning administration route, which was left to the discretion of the clinical investigator, based on patients’ chemotherapy schedules and hospitalization conditions in each institution. Further research is needed to develop evidence-based criteria for assigning administration route, and to compare and analyze response to RHuEPO treatment by administration route in pediatric cancer patients.
This study is the first clinical trial using Cuban recombinant human erythropoietin (ior®EPOCIM) to treat anemia in pediatric cancer patients, thereby providing baseline data for future research. It is also contributes to the literature in an area on which few studies have been published internationally. The study was particularly important in Cuba, from a clinical standpoint, as it gave Cuban oncologists and hematologists—especially those in pediatrics—an opportunity to gain new experience in the use of ior®EPOCIM.
Based on the results obtained, introduction of ior®EPOCIM in all pediatric oncology and hematology services in the country was recommended.
CONCLUSIONS
The Cuban ESA, ior®EPOCIM, proved effective in correcting anemia and reducing transfusion requirements in pediatric cancer patients. It also proved to be safe and well-tolerated, as evidenced by the low frequency of adverse events associated with treatment.
ACKNOWLEDGMENTS
We gratefully acknowledge the collaboration of all the researchers who participated in the patient inclusion phase of the study and assisted in data collection. Without them, completion of the study would not have been possible.








