INTRODUCTION
Globally, the burden of cancer continues to rise, as a result of population aging[1] and the spread of cancer-related unhealthy behaviors.[2] Cancer is the first cause of death in high-income countries and the second in developing countries.[3] The problem is equally serious in Cuba. Galán described trends in cancer incidence (1990 to 2003) and mortality (1990 to 2007) in Cuba, reporting an increase in age-standardized rates of both.[4] In 2012, cancer deaths in Cuba rose to 22,532, making it the leading cause of death. For more than a decade, cancer has also been the leading cause of premature death in Cuba, first in the population aged 15–79 years.[5]
Worldwide, the complex cancer situation calls for better strategies for prevention, early detection and effective treatment. To help guide, monitor and evaluate these strategies, the magnitude of the problem must be quantified and its progression tracked over time. To this end, concerted efforts have been made to estimate the global burden of cancer.[3,6,7] The indicators most often used at one time were incidence, mortality and survival. However, none of these is perfect, and analyzing their estimates separately can be complicated and not very helpful in setting priorities and allocating resources.[7]
The 1990 Global Burden of Disease (GBD) study addressed this problem by introducing a new measure, disability-adjusted life years (DALY), a measure of time that integrates years of life lost due to premature mortality (PYLL) and years lived with disability (YLD).[8] Since then, numerous studies have used DALYs to assess cancer burden.[8] The principal advantages of DALY over other epidemiological indicators is its greater feasibility as a results indicator in cost-effectiveness studies, since it provides a single measure for mortality and morbidity.[9] Unfortunately, it has not been widely applied in Cuba.
In our earlier research using DALY to calculate the burden of 16 cancer types in Cuba and its provinces for both sexes and all age groups in the period 1990–2002, three sites of cancer related to the female reproductive system (breast, cervical and endometrial cancers) were among the six in women with the highest burden, and their rising trends made them particularly noteworthy.[10–12] Some of these, in particular cervical cancer, tend to affect younger people. According to Galán, cervical cancer was the most frequent cancer in women aged 20–39 years in 1990–2003.[4] In 2012, cervical cancer was the most frequent cause of cancer deaths in women aged 20–39 years and breast cancer was the most frequent cause of cancer deaths in women aged 40–59 years.[5]
This situation introduces another unfavorable factor in an already complicated panorama: potential harmful effect on women’s reproductive capacities. Today women in many countries more frequently postpone childbearing, until other life goals have been met. This delay makes it more probable that cancer will be diagnosed before they have children, or the number of children they want.[13]
An estimated 75% of young women diagnosed with cancer want to have children in the future.[14] Recent studies emphasize the negative effect on their quality of life when cancer implies losing reproductive capacity—a loss that in some cases may be more psychologically devastating than the cancer diagnosis itself.[13–15] Such considerations led us to ask whether the negative trend of cancer burden in sites related to the female reproductive system, identified by the 1990–2002 study in all age groups,[11] would also be observed if only women in childbearing years were included. Thus, we developed the present study, which provides the first estimated DALY for breast and reproductive system cancers in Cuban women of childbearing age.
METHODS
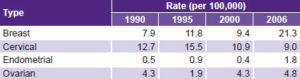
A descriptive epidemiological study was conducted with national-level data on the burden of four types of cancer (breast, cervical, endometrial and ovarian) in 1990, 1995, 2000 and 2006. ICD-9 classifications were used for the first three years and ICD-10 for 2006 (Table 1). The year 2006 was selected as the last year because it was the most recent for which reliable incidence data were available during the study design phase.
Given the trend among Cuban women to postpone pregnancy to later years, we defined childbearing age as 15–44 years, although the upper limit is later than some researchers suggest.[13]
Table 1: Cancer types studied
*cancer of endometrium and unspecified parts of uterus
Data sources and analysis Data on incidence, prevalence and mortality for each of the four cancer types for the age–sex group studied were retrieved from Cuban national registries: underlying cause of death and mortality for five-year intervals from mortality databases at the Ministry of Public Health’s National Statistics Division (DNE-MINSAP, the Spanish acronym); estimated life expectancies by five-year age group from Cuba’s National Statistics Bureau (ONE, the Spanish acronym);[16,17] and incidence (number of cases) for each cancer type by age group from the National Cancer Registry. SPSS program version 19 was used for data management.
Prevalence was calculated by applying the formula: prevalence = incidence × 5, based on consultations with experts at the Institute of Oncology and Radiobiology (INOR, the Spanish acronym); while it may not be the most precise way to determine prevalence, it was the only feasible option. A more precise method would be to multiply incidence by survival, using specific survival estimates for each type of cancer, but national-level data on survival for all four types of cancer were not available. Wide country-specific variations in survival make it inappropriate to use estimates from other contexts.[7]
DISMOD II was used to analyze data, an application that estimates six internally consistent epidemiological indicators for which values (from registries) must be introduced for at least three. The six are: incidence, prevalence, mortality, remission, average age at onset, and average duration.[18] The application was developed in the framework of GBD 1990[8] to obtain more reliable estimates (than from national and regional registries) to calculate YLD.
Premature mortality burden calculation Total deaths in Cuba of women aged 15–44 years were considered in which underlying cause of death was attributable to one of the four cancer types studied. PYLL was calculated by the usual method for burden of disease studies;[8] applying estimated life expectancies by five-year age group. PYLL rates were calculated per 100,000 women aged 15–44 years. In addition, mean PYLL per each death was calculated.
Morbidity burden (YLD) calculation This measure was calculated as the product of incidence (number of cases), average duration (both obtained as outputs of the DISMOD II program) and disability weight for each cancer type. Disability weights are values between 0 and 1, in which 0 indicates perfect health and 1 death. Methods to obtain disability weights in different disease burden studies have been extremely rigorous and are described in various publications.[8,19,20] Disability weights used were from GBD 1990,[8] with the same adjustments made in the 1990–2002 Cuban study.[11] The disability weights were: breast (0.24); cervical (0.25); endometrial (0.29); and ovarian (0.25). YLD rates per 100,000 women aged 15–44 years were calculated.
Disease burden calculation This is the sum of PYLL and YLD. DALY rates per 100,000 women aged 15–44 years were calculated, both crude and age-standardized to the median population for the four years studied.
Ethical considerations All data in the study were retrieved from registries, and data management procedures ensured confidentiality of individual patient information. The study was approved by the National Endocrinology Institute (INEN, the Spanish acronym) ethics committee.
RESULTS
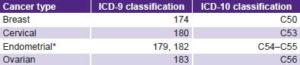
Table 2 shows PYLL rates (per 100,000 women aged 15–44 years) for each type of cancer for the four years studied. Breast cancer and cervical cancer presented the highest PYLL rates, and also the sharpest increases over the period. Although also presenting rising PYLL levels from 1990 to 2006, endometrial and ovarian cancers showed smaller increases, especially endometrial cancer.
Cervical cancer presented a greater increase than breast cancer. In fact, cervical cancer, with a lower PYLL rate than breast cancer in 1990, surpassed it in 2006. A slower rise (which could be interpreted as practically stable) for cervical cancer occurred from 2000 to 2006. The PYLL rate for breast cancer climbed steadily throughout the entire study period.
Table 2: PYLL in women aged 15–44 years by cancer type, Cuba
PYLL: potential years of life lost due to premature mortality
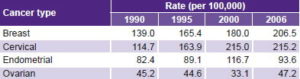
Table 3 shows average PYLL lost for each death. An increase can be seen from 1990 to 2006 for the four types studied, with greater increases for breast and cervical cancer. This trend over the entire study period is not consistent from 2000 to 2006, when slight drops (or stabilization) of this indicator can be observed for the four sites.
Table 3: Mean PYLL per death in women aged 15–44 years by cancer type, Cuba
PYLL: potential years of life lost due to premature mortality
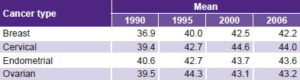
Table 4 shows YLD rates per 100,000 women aged 15–44 years by type for the 4 years studied. Increased YLD can be seen for three of the four cancer types, with the exception of cervical cancer. Breast cancer shows the greatest YLD rise, peaking in 2006 with the highest value among all types of cancer and years studied (21.3 per 100,000). Endometrial cancer showed an increase similar to breast cancer’s, although with much lower values; while ovarian cancer showed a more modest rise. Endometrial cancer showed the least YLD during the four years studied.
Table 4: YLD in women aged 15–44 years by cancer type, Cuba
YLD: years lived with disability
Table 5 presents crude and age-standardized DALY rates per 100,000 women aged 15–44 years by cancer type for the 4 study years. Breast and cervical cancer are the two types with highest DALY rates, both crude and standardized, in all four years, with a net upward trend over the period, greater for cervical cancer. Breast cancer crude DALY rates continued to rise steadily during the four years studied, but cervical cancer showed a modest decline from 2000 to 2006. Age-standardized DALY rates for both sites declined between 2000 and 2006. Endometrial cancer and ovarian cancer were in third and fourth place, respectively; for both, crude rates increased but age-standardized rates decreased.
Table 5: DALY in women aged 15–44 years by cancer type, Cuba
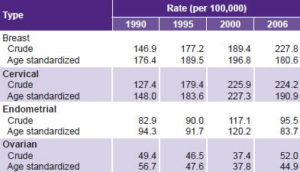
DALY: disability-adjusted life years
DISCUSSION
The unfavorable trend in mortality indicators (PYLL rate and PYLL per death) in our study—especially for breast and cervical cancers—are consistent with results reported in other Cuban national studies. The 2012 DNE-MINSAP report found cervical cancer to be the first cause of cancer deaths in women aged 20–39 years (3.1 per 100,000), and breast cancer to be the first in women aged 40–59 years (26.6 per 100,000).[5] These statistics are consistent with our observation that breast and cervical cancers generated the highest premature cancer mortality in women aged 15–44 years.
The results of our research are similar to those of the 1990–2002 study, which included the same types of cancer (but all age groups). It also found an increase in PYLL rates in the four sites, with breast cancer still the highest generator of premature deaths; in second place was endometrial cancer during the 1990–1995 period and cervical cancer from 2000 to 2002. Ovarian cancer remained in fourth place in all four years of the earlier study.[11]
The panorama described for Cuba in relation to these types of cancer is similar to the global picture. In 2010, breast and cervical cancers caused 4.2% of deaths in women aged 15–49 years worldwide,[21] placing them in sixth and seventh place, respectively, among all causes of death in this age group. Comparable Cuban data specific to this age group are not available, but the two types of cancer caused 2.2% of cancer deaths among women of all ages in 2012.[5] GLOBOCAN 2012, an online database of the International Agency for Research on Cancer (IARC), revealed surprising cancer patterns in women, warning of the imminent need to develop and improve measures aimed at control and prevention of breast and cervical cancers worldwide.[3]
The rise in mean PYLL per death is another negative trend observed, coinciding with results from the previously cited report for 1990–2002.[11] To a certain degree, however, it is offset by the fact that between 2000 and 2006, a downward trend, albeit modest, was observed in this indicator for three of the four types of cancer (breast, cervical and endometrial cancers), while ovarian cancer rates remained stable.
YLD rates observed are inconsistent with those in the 1990–2002 study[11] in which breast, cervical and ovarian cancers were rising and endometrial cancer was holding steady. This may be due in part to differences in the age groups studied.
GBD 2010 (conducted for WHO by a group of researchers from 50 countries coordinated by the Institute for Health Metrics and Evaluation) reported a 35.8% global increase in YLD rates attributable to cancer (all types) from 1990 to 2010. (It should be borne in mind in interpreting all comparisons that GBD 2010 data are age-standardized rates for all ages.) Three of the cancer types we studied increased: breast cancer by 37%, endometrial cancer 11.2% and ovarian cancer 18.3%. Cervical cancer decreased by 14%.[22] Breast and cervical cancers were the greatest contributors to disease burden (DALY) in our study, crude rates of which rose for all four cancer types in our study as well, as in the 1990–2002 study, despite the differing age groups included.[11] The fact that this trend disappeared with age standardization reflects the influence of population aging over the study period. Some discrepancies are observed with GBD 2010, which reported worldwide increases in DALY rates from breast cancer (4.5% vs. our 2.4%) and ovarian cancer (6.1% vs. our decrease of 20.8%) between 1990 and 2010, and decreases for cervical cancer (11% vs. our increase of 28.9%) and endometrial cancer (3.7% vs. our decrease of 11.2%).[23] GBD 2010 reported breast and cervical cancer among the top 25 contributors to PYLL, with increases of 30% and 3%, respectively, from 1990,[24] much lower than the PYLL increases we found of 48.6% for breast cancer and 87.6% for cervical cancer.
Breast cancer Cuba’s Comprehensive Cancer Control Program (PICC, the Spanish acronym) includes actions aimed at early detection of breast cancer, which has a higher survival rate when diagnosed early.[25] The increase in breast cancer mortality reflected in the PYLL rates and mean PYLL per death we observed should be analyzed by PICC decisionmakers and investigated further to understand why early detection efforts are not having the desired effect.
An increase of 14% in breast cancer deaths worldwide was reported from 2008 to 2012, making it the first cause of cancer deaths in women (522,000 deaths in 2012).[3] Although breast cancer incidence has increased in most of the world, obvious inequities between rich and poor countries are observed in mortality. Developed countries have the highest incidence rates, but mortality rates are higher in developing countries because of limited access to early detection and effective treatments.[3] The decline in mortality in more developed countries is largely related to the use of mammography screening, which can reduce breast cancer mortality rates in women aged 50–69 years by 20 to 35% (at 14 years of followup).[26] Globally, breast cancer 5-year survival rates vary from 9 in 10 women diagnosed in stage 1, to 1 in 10 diagnosed in stage 4.
The rising YLD rates we found for breast cancer, much more evident between 2000 and 2006, are consistent with DNE-MINSAP’s report of an increase in age-standardized incidence from 32.9 to 39 per 100,000 between 2000[27] and 2006.[28] Worldwide, 1.7 million women were diagnosed with breast cancer in 2012, a 20% increase over 2008. Among women, it is the cancer with the highest incidence in 140 of 185 countries, accounting for one of every four cases of cancer diagnosed.[3]
A rise in mortality and morbidity burdens attributable to breast cancer in the study period corresponds to an overall increase in DALY rates reported by WHO for this type of cancer between 1990 and 2010,[23] although for Cuba, the increase is proportionally greater. The decrease in age-standardized rates noted between 2000 and 2006 may indicate the beginning of longer-term reduction in breast cancer burden; verifying this will require ongoing DALY monitoring.
Cervical cancer In terms of premature mortality, although we saw increases in both PYLL rates and mean PYLL per death from cervical cancer, it is important to note the trend from 2000 to 2006 in the two indicators. PYLL rose slightly, and YLD fell, again not by much, but striking against the background of sharply rising rates of the two indicators in previous years. These results could indicate the beginning of a deceleration in the rising mortality rate from cervical cancer. This trend should be monitored in the future (applying the same indicators).
In 2012, cervical cancer was the main cause of cancer deaths in Cuban women aged 20–39 years.[5] This situation acquires particular importance when we consider that early detection leads to a high rate of cure. In fact, the worsening trend in mortality shown by this and other studies and reports[4,5] could be related precisely to late diagnosis.
For early detection efforts to produce appreciable results, they must cover at least 80% of the target population. Evidence in Cuba shows that the number of women screened for cervical cancer by cytology has fluctuated in recent years[5] and is below the percentage needed to reduce mortality rates.[29] Considering that PICC includes actions to ensure access to screening in primary health care,[25] it is clear that women are not sufficiently aware of the need for cytology testing at the recommended intervals. To address this, more proactive primary care approaches are needed, in concert with other organizations and institutions, to implement intensified education and recruiting programs.
Cervical cancer is the fourth most common type of cancer in women worldwide, with 528,000 new cases diagnosed annually. The situation is most alarming in less-developed regions, where approximately 70% of the global burden of this cancer is concentrated. It is the fourth leading cause of cancer-related deaths in women worldwide (266,000 deaths in 2012), with significant differences among countries.[3] Its effects are devastating, with extremely high human, social and economic costs, affecting women early in their adult lives.[30] Introduction of HPV vaccine has been a major advance in preventing cervical cancer. Unfortunately, it is very expensive for developing countries, hence the need to insist on screening programs.[31] Lack of access to preventive treatment (vaccines) and effective alternative therapies, and the absence of organized screening programs, can explain the inequities in cervical cancer affecting less-developed regions. Currently, sub-Saharan Africa bears the largest burden, with 34.8 new cases per 100,000 women diagnosed annually and 22.5 deaths per 100,000 women, compared to 6.6 and 2.5 per 100,000, respectively, in North America (Canada and the USA).[3] IARC has insisted on the need for effective control strategies: essentially the HPV vaccination combined with well-organized national screening programs,[30] although without comment on how low-income countries are to finance such efforts.
The downward trend in the YLD rate for cervical cancer observed in 2000 and 2006 is consistent with reduced cervical cancer incidence reported by the DNE-MINSAP (with age-standardized rates of 20.1 in 2000 and 16.6 per 100,000 in 2006)[27,28] and by WHO for 1990–2010.[22]
This YLD decline for cervical cancer is accompanied by a rise in the same indicator for endometrial cancer. Therefore, we cannot rule out a coding artifact: the apparently positive trend of the burden of morbidity for cervical cancer in 2000 and 2006 may be related to inclusion of nonspecific uterine parts in the category of endometrium, which could lead to cases of cervical cancer being mistakenly categorized as endometrial cancer, whether due to late diagnosis or incomplete case reports.
Galán reported that from 2001–2003 cervical cancer was the most common cancer in Cuban women aged 15–44 years,[4] attributing this to sexual behavior, specifically early exposure to sexually-transmitted infections, particularly HPV, the main etiological factor.[31–33] In our study, although the YLD rate fell, the DALY rate for cervical cancer rose in the period, largely due to the mortality component; however, a trend toward stabilization of crude rates was observed between 2000 and 2006, and the age-standardized rate decreased.
Endometrial cancer The upward trends we observed in PYLL rates and mean PYLL per death for endometrial cancer are striking. Considering that deaths from endometrial cancer begin to climb after age 60,[5] the rise in premature mortality from this type of cancer in women aged 15–44 years urgently calls for more detailed analysis.
The 1990–2002 study also showed a rise in PYLL rates for endometrial cancer. Cuban provinces with relatively low rates of premature mortality from cervical cancer also had high rates of endometrial cancer mortality.[11] This was especially true in some eastern provinces. Study authors posed the same plausible explanation for this phenomenon as stated above for a period in our study: the possibility that deaths from cervical cancer were mistakenly categorized as deaths from endometrial cancer. This could be due to late diagnosis when it is no longer possible to specify the part of the uterus originally affected, and/or incomplete cause-of-death reports. This situation should be the focus of further study.
Mortality rates for endometrial cancer increase linearly with age, peaking in women aged ≥85 years; there is little variation from one country to another, despite lower incidence in less-developed countries, where low survival rates keep mortality rates closer to those seen in high-income countries.[3]
Endometrial cancer is four times more prevalent in developed than developing countries, the opposite of cervical cancer.[3] The increase in YLD for endometrial cancer is in line with the rise in the incidence rate (from 2000 to 2006) reported by the DNE-MINSAP[27,28] as well as that described by WHO for the period 1990–2010.[22] The increase in crude DALY rates observed from 1990 to 2006 in our study does not correspond with the global decrease of 3.7% reported by GBD from 1990 to 2010.[23]
Ovarian cancer Ovarian cancer showed the lowest PYLL rates of the four types studied, with a slight increase during the period. The increase in mean PYLL per-death rate is similar to that for the other three cancer types. These results are in keeping with those from 1990 to 2002 in Cuba.[11] Other Cuban national studies do not report ovarian cancer as among those causing high mortality.[4,5]
The slight increase in YLD for ovarian cancer is consistent with rising incidence from 2000[27] to 2006[28] as reported by DNE-MINSAP, as well as the global trend for 1990–2010.[22]
Globally, ovarian cancer accounts for 4% of cancers in women, with wide variations among countries.[3] The rise in crude DALY rates in this period is consistent with global trends,[23] but age standardization produced a decrease between 2000 and 2006. This difference highlights that fact that population aging can increase burden (evident in crude rates) even with declining risk (reflected in age-standardized rates).
Cancer risk The age group studied lends special significance to the results of this research. Increased crude DALY rates for these cancers (and in age-standardized rates for breast and cervical cancer), with the resulting rise in burden of disability and mortality among women aged 15–44 years, leads to women abandoning or postponing personal and social aspirations at this time of life; of particular importance is impaired fertility resulting from cancer diagnosis and treatment. Significant scientific evidence explains how different cancer treatments can impair reproductive capacity,[34–39] with the resulting consequences for individuals, families and society.
The growing effects of cancer in Cuba, as seen in recent decades, are fundamentally associated with a rise in behavioral risk factors[40] and population aging.[41] Cuba’s national public health system has already been focusing substantial efforts and resources on cancer control, as specified in the PICC.[25] The results of this study, however, attest to the need to refocus, intensify and monitor outcomes of PICC strategies.
This is the first nationwide study in Cuba to quantify DALY rates attributable to breast and reproductive system cancers in Cuban women of childbearing age. The study may have the added benefit of helping to inform the scientific community and decisionmakers about a poorly utilized indicator in our country. Data on mortality and cancer rates were taken from national registries and are therefore limited, so a certain margin of error is inevitable; a level of subjectivism is inevitably associated with assigning severity weights, regardless of the rigor with which data were originally obtained for the GBD study of 1990;[8] and the method for calculating prevalence values (the only feasible option) was not precise.
CONCLUSIONS
The use of a different method (DALY rates) from those usually applied in Cuba helped reveal a negative trend, if very unequal, in overall burden of the four sites of cancer studied in women of childbearing age from 1990 to 2006. These results should provide feedback, in the first instance, to the PICC; by providing evidence of the need to reinforce actions aimed at prevention and active screening, especially for breast and cervical cancer, where early detection has a proven positive impact on survival.








