ABSTRACT
INTRODUCTION Improved recovery protocols were implemented in surgical specialties over the last decade, which decreased anesthetic and surgical stress and the incidence of perioperative complications. However, these recovery protocols were introduced more slowly for cardiac surgeries. The most frequent complications in cardiac surgery are related to patient clinical status and the characteristics of the surgical procedures involved, which are becoming more varied and complex every day. The first version of the enhanced recovery program for cardiac surgery was published in 2019, but its recommendations were based on only a few studies, and scant research has evaluated its implementation. Randomized and controlled clinical trials for these protocols are scarce, so research that summarizes the results of studies with other methodological designs are useful in demonstrating their benefits in cardiovascular surgery services in Cuba and in other limited-resource settings.
OBJECTIVE Estimate the effectiveness of improved recovery protocols in the perioperative evolution of patients undergoing cardiac surgery.
METHODS We performed a systematic review and meta-analysis according to the guidelines of manual 5.1.0 for reviews of the Cochrane library. We included observational and quasi-experimental studies published from January 2015 through May 2020 that compared enhanced recovery protocols with conventional treatments in patients older than 18 years, and used a quality score to evaluate them. We used the following sources: the Cochrane Library, PubMed, LILACS, SciELO, EBSCO, Google Scholar, Web of Science, Clinical Key, ResearchGate and HINARI. The following keywords were used for the database searches in English: ERAS, protocols and cardiac surgery, enhanced recovery after cardiac surgery, ERACS, clinical pathway recovery and cardiac surgery, perioperative care and cardiac surgery. We used the following search terms for databases in Spanish: protocolos de recuperación precoz and cirugía cardiaca, protocolos de recuperación mejorada and cirugía cardiaca, cuidados perioperatorios and cirugía cardiaca, programas de recuperación precoz and cirugía cardiovascular. Methodological quality of included investigations was evaluated using the surgical research methodology scale. Meta-analyses were performed for perioperative complications, intensive care unit and hospital stays, and hospital readmission within 30 days of surgery. We calculated effect sizes of the interventions and the corresponding 95% confidence intervals. We used mean differences and confidence intervals for continuous variables, and for qualitative variables we calculated relative risk (RR). Random effects analysis was used. Heterogeneity of the studies was assessed using the Q statistic and the I2 statistic.
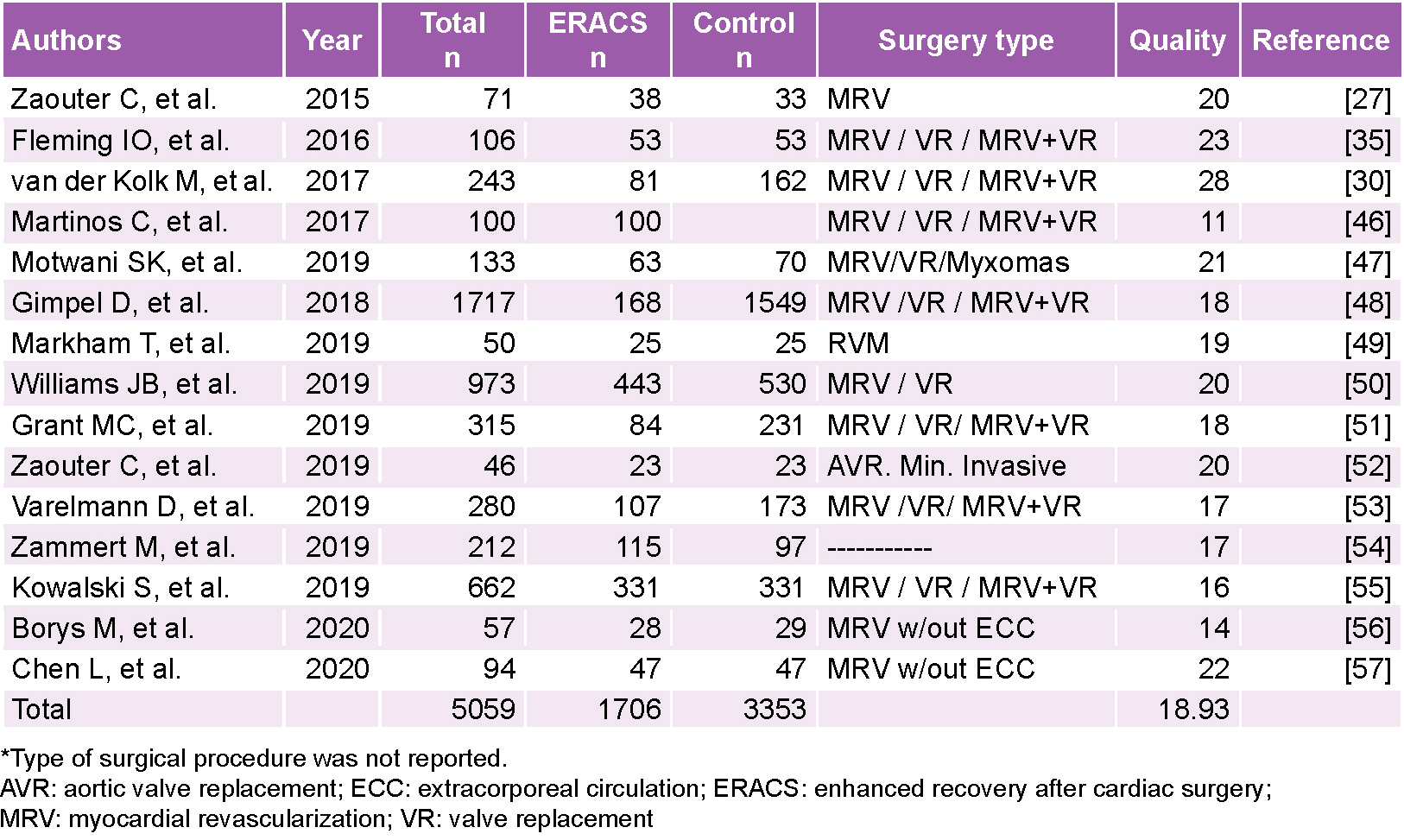
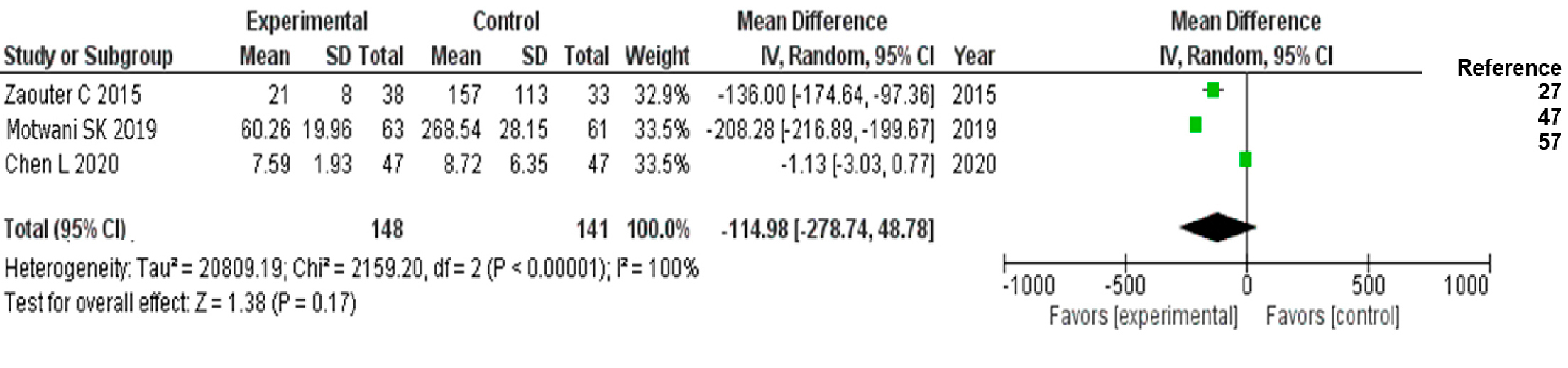
RESULTS We selected 15 studies (a total of 5059 patients: study group, n = 1706; control group, n = 3353). The average quality score for the 15 articles included was 18.9 (out of a maximum of 36 according to the scale) and 66.6% had a score ≥18. With improved recovery protocols in cardiac surgery, the incidence of perioperative complications decreased (RR = 0.73; 95% CI 0.52–0.98) as did hospital readmission within 30 days after surgery (RR = 0.51; 95% CI 95% CI: 0.31–0.86). Differences in extubation time, hospital stay and length of stay in intensive care units were less marked, but always favored the group in which the enhanced protocols were implemented.
CONCLUSIONS Improved recovery protocols in cardiac surgery increase quality of care evidenced by reductions in perioperative complications and decreased incidence of hospital readmission in the month following surgery.
KEYWORDS Enhanced recovery after surgery, rehabilitation, perioperative care, thoracic surgery, cardiac surgical procedures, systematic review, meta-analysis, Cuba
INTRODUCTION
In the last decade, improved recovery protocols were introduced in the surgical clinics of various specialties, which decreased anesthetic and surgical stress, as well as incidence of perioperative complications and morbidity; but their use in heart surgery has been slower despite the obvious advantages. In cardiac surgical procedures, the most frequent complications are related to patient clinical status, including comorbidities, and to increasingly complex and varied surgical procedures. The multimodal, multidisciplinary and continued-care approach of these protocols—which are applied before, during and after surgery—aim to improve quality of care and perioperative evolution, and to aid in early recovery.[1]
IMPORTANCE This study provides evidence pointing to benefits of improved recovery protocols in cardiac surgery, which may lead to their implementation in Cuban heart surgery units and those of hospitals in limited-resource settings.
Patients who undergo cardiac surgery are exposed to events and procedures that can become risk factors for increased morbidity and mortality, including but not limited to: progressive deterioration of nutritional status due to decreasing daily intake and preoperative fasting; anticoagulation procedures during the intraoperative period; prolonged periods of aortic clamping and cardiac arrest; extracorporeal circulation including the potential development of an inflammatory response syndrome; blood transfusions; intensive pharmacological support or mechanical support for low-output syndrome; and late postoperative nutritional support.[1–3] Improved recovery protocols propose comprehensive treatment with actions that cover the entire perioperative period and are designed to ameliorate the negative effects of these factors, and hence they are recommended for implementation in cardiac surgery units.
In 2002, Henrik Kehlet introduced the concept of enhanced recovery protocols (ERAS), and from his work the international non-profit society Enhanced Recovery After Surgery Society (ERASS) was created.[3–6] These programs were applied first in colorectal surgery, and later extended and adapted to other surgical specialties.[4,6–11] The main objective of ERAS protocols is that patients arrive at the surgical procedure in the best clinical conditions possible and that they remain so during and after surgery until discharge via preoperative, intraoperative and postoperative interventions.[7,8,11–15]
ERAS was slow to be introduced into cardiac surgery compared to some other surgical specialties due to the complexity of procedures, differences in conditions required for each intervention, and wide diversity of patient clinical characteristics.[3,16] The first enhanced recovery programs in cardiovascular surgical procedures were the so-called fast-track and ultra-fast track programs, introduced in the 1990s.[17–19] These proposed shortening the duration of orotracheal extubation and postoperative ventilation mechanics, which are risk factors for respiratory complications, as well as shortened stays in hospitals and intensive care units (ICUs). But these actions were focused on a single stage of the perioperative period and were not multidisciplinary. In cardiac surgery, such fast-track and ultra fast-track programs are not applied to all cardiac surgical procedures or to all patients.[17,19–26]
Between 2017 and 2019, publications on the results of ERAS programs in cardiac surgery increased.[6,14,19,23,27–33] World leaders in the specialty recognized the need to adapt the original ERAS programs to cardiac surgery patient characteristics and to each type of intervention, and to generalize a protocol based on the best scientific evidence.[2,14,34,35] The first cardio-surgical symposium for development, evaluation and control of enhanced recovery protocols was held in 2017, whereas ERAS experts published the first ERAS guidelines for cardiac surgery in March 2019,[34,36,37] collectively known as ‘ERACS protocols or guidelines’.
These ERACS guidelines have the following characteristics: in the preoperative stage, they propose to educate patients and family members, stratify and control nutritional status, estimate blood glucose levels using glycosylated hemoglobin, eliminate risk factors (tobacco and alcohol), treat infections with prophylaxis, administer carbohydrates two hours before surgery, detect kidney dysfunction early and decrease fasting time (six hours for solids and two to four hours for clear liquids). For the intraoperative period, they propose performing antifibrinolytic therapy with tranexamic acid or Epsilon aminocaproic acid, using multimodal anesthetic and analgesic techniques involving minimal opioids, administering fluids according to hemodynamic variables, controlling hypothermia, maintaining glycemic control, implementing prophylaxis of acute kidney injury and of infections, and using a plate for rigid sternal fixation. For postoperative recovery, they recommend intensively controlling blood glucose levels via continuous infusion, removing dressings from wounds at 48 hours, maintaining thromboprophylaxis, preventing hypothermia, treating pain with minimal use of opioids, stratifying and controlling postoperative delirium, treating acute kidney injury prophylactically, and extubating within the first 6 hours after surgery.[37]
Despite progress in introducing these programs for heart surgery, the authors of the first guidelines concluded that there was not enough published research on the subject, and not enough sound evidence such as that provided by randomized controlled clinical trials (RCTs) and systematic reviews or meta-analyses. Guidelines were issued when there were enough studies to support the introduction of therapeutic measures and diagnostic means.[37]
Evidence-based clinical practice is related to better quality of patient care and improvements in major hospital indicators, and so systematic reviews have gained more followers than detractors and have come to be seen in recent decades as essential tools in developing evidence-based medicine. The validity of individual studies is increased through systematic reviews and areas of controversy are identified where it is necessary to update information and build consensus.[38,39]
At the cardiac unit of the Hermanos Ameijeiras Clinical–Surgical Hospital (HHA) in Havana, Cuba, the first RCT (retrospective record dated 06/09/2012, code RPCEC00000131) was carried out on enhanced recovery in cardiac surgery, with fast-track and multimodal anesthetic methods (association of spinal regional anesthetic techniques with general anesthesia) in myocardial revascularization surgery without extracorporeal circulation. As a result of this RCT,[18] our practice experienced better results during perioperative analgesia, lower doses of systemic opioids were used, the time of mechanical ventilation in the postoperative period was reduced to less than four hours, and incidence of perioperative complications and postoperative stays in hospitals and ICUs decreased. This was the first step in implementing anesthesia strategies based on the best clinical evidence for optimizing patient recovery.[40]
Controversies persist on the benefits of multimodal anesthesia methods that include spinal regional anesthetic techniques in cardiac surgery, because some studies show that these methods do not reduce morbidity in the 30 days following surgery.[41] The authors of the first international version of the ERACS protocol stated that these methods require further evidence and expert evaluation before formal inclusion in the recommendations.[37]
Currently, data is scarce on the benefits of introducing improved recovery protocols in the perioperative clinical practice of cardiac surgery, so we set out to estimate the effectiveness of applying these protocols in the perioperative evolution of patients older than 18 years of age undergoing cardiac surgery, compared with the conventional protocol, based on the primary results of perioperative complications, length of stay in ICUs and hospitals, and hospital readmission within 30 days after the procedure, through a systematic review of observational and quasi-experimental studies, and a meta-analysis.
These programs are useful in focusing on surgical patient care in a comprehensive manner and improving patient care quality by establishing best practices based on documented evidence.
METHODS
This study is a first approximation based on observational and quasi-experimental methodological designs. We carried out a systematic review according to the recommendations outlined in version 5.1.0 of the Cochrane Handbook for Systematic Reviews of Interventions, and the evaluation criteria of the international guide “Preferred Reporting Items for Systematic Reviews and Meta Analyses” (PRISMA).[42,43]
The protocol for this systematic review has been approved by HHA’s Scientific Commission (version 0.0, number 2657, May 2018), but it was not registered in electronic databases with national or international access, as is suggested by the PRISMA evaluation guides.[44]
Different meta-analyses were performed for variables of interest whose data were available in three or more of the included studies and whose summary measures were compatible for processing with the EPIDAT 3.1 and Review Manager 5.3 (RevMan 5.3) programs, because all studies did not include the same variables and they needed to be grouped to evaluate those that were both available and of interest.
Search strategy for identifying studies We use the Cochrane Library, PubMed, LILACS, SciELO, EBSCO, Google Scholar, Web of Science, Clinical Key, ResearchGate and HINARI as sources for studies in humans published from January 2015 through May 2020, in both Spanish and English.
The following search terms were used: For databases in English, ERAS; protocols and cardiac surgery; enhanced recovery after cardiac surgery; ERACS; clinical pathway recovery and cardiac surgery; perioperative care and cardiac surgery. For databases in Spanish: protocolos de recuperación precoz and cirugía cardiaca; protocolos de recuperación mejorada and cirugía cardiaca; cuidados preoperatorios and cirugía cardiaca; programas de recuperación precoz and cirugía cardiovascular.
The search syntax in PubMed, the database that contributed the most references, was as follows:
- Enhanced recovery AND cardiac surgery
- Cardiac surgery AND perioperative care
- Heart surgery AND clinical pathway
- Perioperative care AND heart surgery
- # 1 or # 2 or # 3 or # 4
During the first stage, we reviewed titles and abstracts of articles with the potential of meeting study requirements that appeared in the abovementioned search engines. In the second stage, we searched and examined the full texts of the articles selected by title and abstract. Two independent evaluators were used in both stages and discrepancies were discussed. We screened the reference lists of selected articles (a ‘search for pearls’) to find studies that might be included in the systematic review. We were unable to contact the authors of articles with incomplete information or who presented their information in the form of graphics. An operational model was designed to select studies that included explicit criteria for collecting information. Search results were processed using Zotero 5.0 for Windows bibliographic reference manager.
Criteria for evaluating studies
Study type
- Observational
- Quasi-experimental
Participants
Patients aged >18 years scheduled for cardiac surgery with or without extracorporeal circulation (ECC)
Intervention
- Enhanced recovery protocols or ERACS protocols
- Conventional protocols
Main outcome measures
Primary or critical outcomes that directly influence decisions
- Perioperative complications
- Length of stay in the ICU
- Length of stay in hospital
- Hospital readmission within 30 days after surgery
- Patient satisfaction
Secondary, important, non-critical outcomes that can influence decisions
- Extubation time
- Administration of inotropic drugs
- Early enteral nutrition
- Early mobilization
- Total water balance
We did not define these results in the methodology, as definitions may differ between studies, and thus for each study reviewed, we used the same definitions as the researchers.
Exclusion criteria RCTs were excluded, as the purpose of this study was to carry out a systematic review of observational and quasi-experimental investigations for which there were no previous reviews. Studies that did not answer the review questions were also excluded.
Data collection and analysis Two observers collected information independently and selected studies according to the established criteria based on intervention type, participants and outcome measures. When there were discrepancies, a third evaluator was consulted until a consensus was reached. This procedure was followed in the order set forth in the search strategy.
Methodological quality This was assessed for each article using the Methodology of Research in Surgery (MINCIR) scale[45] validated for studies of therapy or therapeutic procedures. This scale consists of three domains: the first assigns scores 1–12 for design type, with the highest score for RCTs, particularly multicenter ones; the second evaluates sample size regardless of the method (or lack thereof) of calculation, and the third is composed of four items, assigning scores of 1–3 to each, which are: quality of the objectives, mention of or justification of the study’s design, sample selection criteria (inclusion and exclusion) and whether or not the sample size is justified. The score’s total is then 6–36 points. The cut-off value for methodological quality was 8 points, because RCTs were not included, and studies were observational and quasi-experimental. Studies that obtained a score ≥18 were assessed as having good methodological quality, and studies with a score ≤17 points were assessed as having poor methodological quality.
Procedures for meta-analysis Magnitudes of the interventions’ effects with their respective 95% confidence intervals (CI) were calculated for the qualitative response variables using relative risk (RR) as a measure of effect, calculated as risk of event in the ERACS group/risk of event in the control group, so that higher risks of the event presenting in the control group (CG) produced RRs lower than would have been the case had the two groups been combined. For quantitative variables, the difference in means between the ERACS group and the CG was used as a measure of effect, so that values <0 implied a favorable effect for the intervention. Random effects analyses were used for all variables, since fixed-effect meta-analyses ignore non-random sources of variation between studies. The heterogeneity of the studies was assessed using Q and I2 statistics. Sensitivity was estimated by the change in the global effect when articles with inadequate or poor methodological quality were eliminated (score ≤17). Publication bias was assessed using the Egger t-test statistic.[42]
RESULTS
The study selection process’ exclusion criteria are shown in a flow chart (Figure 1).