Mortality studies based on the causes of death listed on death certificates are not common in international literature, and those that exist generally only take into account the underlying cause of death.[12,18] Studying the underlying cause of death provides important information, but underestimates the implication of chronic conditions, which undoubtedly are among the many influencing factors in the death. This is especially the case for SAD, which are generally present in patients with multiple comorbidities.[18,19]
Since the mid-20th century, mortality studies that analyze multiple causes of death in chronic disease patients have been considered important for gaining new insights into disease prevention. [19,20] In the last decade, there have been studies that use this methodology in SAD mortality.[18,20–26] However SAD mortality studies in developing countries are scarce, and have not had the impact necessary to impart the importance of SADs as an urgent health issue or to implement health policies designed to reduce mortality.[9,27,28] There are few mortality studies in Cuba that use multiple-cause-of-death analysis, but none of them are related to SADs.[29–31]
In this paper, we discuss the relevance and utility of using multiple-cause-of-death analysis for estimating SAD mortality in Cuba and developing countries with reliable data sources in an effort to encourage healthcare programs and policies to acknowledge the impact of SADs.
DEVELOPMENT
SAD in policy and practice In formulating health policies and programs, decisions must be evidence-based, requiring reliable and timely information to assess the magnitude of the diseases within populations and impacts of the programs designed to eliminate or control them, and to establish research priorities accordingly. Mortality registries are the standardized sources of information most commonly used for these purposes.[16,32]
In high-income countries like the United States and Sweden, cardiovascular diseases are the leading cause of death, followed by cancer. However, deaths from diseases deemed ‘rare’ are often ignored. Among these diseases are SADs, which are substantial contributors to premature death.[17] In Cuba, cardiovascular diseases have also been the leading cause of death for several years, but reports from the National Medical Records and Health Statistics Bureau in 2008–2010 placed “systemic connective tissue disorders” among the top 10 causes of death for women aged 15–34 years,[33] and the total deaths attributed to these diseases in 2009–2019 were 1419 for both sexes (84% female).[34] The age group in which those deaths were clustered reinforces the importance of properly diagnosing and treating SADs and of addressing them in health system policies.
A few years ago, SAD were not considered ‘fatal,’ and it was thought that they did not increase short-term mortality, but evidence indicates that mortality may be up to four times higher in SAD patients than in the general population (SMR from 1.44–4.8).[11,12,14,18] One of the reasons low SAD mortality rates are reported in comparison to other diseases is underestimation, since SAD deaths are not reported on death certificates, or at least, not as underlying causes of death—the disease or injury that began the chain of pathological events that directly led to death.[15,16,18,22,35]
Thus, even though these diseases may represent an important burden on individual and population health, they are not recognized as such by many countries when drafting health policies, or determining priorities for research and education.[15,16,22] In response to these deficiencies, WHO promoted 2000–2010 as the ‘Bone and Joint Decade’ in an effort to raise awareness about SADs and decrease their impact on health.[16,36]
Some countries and regions have adopted health policies addressing SADs, but they are few and far between. In response to WHO’s call, the Presidency of the Council of the European Union held a conference in October 2010 on rheumatic and musculoskeletal diseases, where it was recommended that SAD be prioritized in crafting health policy agendas, and that funding for research in EU member countries be increased accordingly. Spain was one of the first countries to design a Comprehensive National Strategy for Rheumatic and Musculoskeletal Diseases,[36] which was supplemented with other regional strategies including the Andalusian Plan for Rheumatic and Musculoskeletal Diseases. It also proposed improving healthcare, increasing SAD awareness and available information, and promoting research and professional training.[36,37]
Several countries have developed SAD-specific policies: for example the National Lupus Public Health Agenda in the United States, which, with 6 priorities, 15 strategies, and 63 recommendations, has achieved both better understanding and management of SLE.[38]
In 2015, the US National Institutes of Health recognized SLE as one of the leading causes of death in young women and increased funding for research into this disease to US$90 million annually. However, despite SLE having an SMR comparable to diabetes mellitus and HIV/AIDS, funding designated for research on diabetes (US$1.01 billion) and HIV/AIDS($US3.17 billion) remains considerably higher.[22]
Countries that already have SAD-specific policies in place relied on information on disease burden, mortality, social determinants of health, the diseases’ temporal and geographic trends, and the use of services related to SAD. That data was supplemented with qualitative studies using evaluations of patients and professionals related to their experiences in patient care.[37,38] In low- and middle-income countries, it is more difficult to obtain this information due to the high cost and organizational and infrastructural difficulties associated with cohort studies and research that provide reliable data on disease burden and mortality, as these countries do not always have services and specialists dedicated to caring for SAD patients.[9,31]
Health authorities in developing countries are hard pressed to fund studies facilitating policy design and implementation for SADs, when forced to dedicate scarce resources to the double burden of diseases that no longer affect high-income countries: communicable diseases plus pressing chronic non-communicable diseases such as diabetes, hypertension, cardiovascular disease, cancer and rheumatic disease.[31,39]
The lack of health policies aimed at improving care for SAD patients in developing countries is evident in the results of a study that analyzed global trends in SAD-related deaths, 2001–2014. In this study, SLE age-standardized mortality rate (ASMR) in Latin America was five times higher than in Europe, and during the 2003–2014 period, there was a significantly increasing trend in SLE ASMRs in Latin America and Asia, while Europe, North America, and Oceania, saw a decreasing trend and ASMRs in Africa remained stable.[1] Hernández Negrín discussed increasing SLE mortality in Latin America and its inverse correlation to country wealth by compiling information from five studies on the topic that obtained their data from mortality registries based on standard WHO death certificates.[27]
Even though health policy deficiencies in recognizing and treating SAD have an important role in SAD severity and high mortality rates, regionalized unfavorable health statistics are due to multiplicity of factors including poverty, malnutrition, low education levels, lack of treatment adherence, adverse perception and non-adaptive behaviors regarding the disease, low clinical suspicion, lack of access to specialized treatments and services, geographic isolation, lack of social support systems, inadequate public policies, and low gross domestic product.[5,9,30,31]
In Cuba, universal health coverage and access provides equitable medical care and epidemiological services to the Cuban population.[40] Yet, despite the existence of rheumatology services designed to diagnose and treat SAD and a Rheumatology Institute conducting research in the field, these diseases are not included in objectives aimed at reducing premature death from select non-communicable chronic diseases (diseases of the arteries and arterioles, cerebrovascular disease, ischemic heart disease, diabetes mellitus, chronic obstructive pulmonary disease, and asthma).[41] Specific public policies for SAD patients are therefore required.
Those policies must be oriented toward improving health professionals’ training pertaining to SADs, increasing human resources for diagnosing and treating SAD patients and researching these diseases, developing health models focused on SAD, establishing standardized national guidelines for SAD clinical management, the systematic collection of clinical and epidemiological data to better understand SAD, raising public awareness, creating support groups for SAD patients and their families, improving access to immunological diagnostic testing, and empowering patients to actively participate in disease management.[9,37,38]
SAD mortality study limitations Medical care for SAD patients is tricky due to diverse (but not uncommon) clinical presentation, and because conventional immunological diagnostic methods can provide similar results for several SADs. Clinical studies arising during the course of SAD treatment are occasionally biased and designed inappropriately, and data quality is not optimal,[15] thus contributing little knowledge related to SAD mortality trends within populations, causes of death, or factors influencing prognoses.[1]
Many SAD studies are based on retrospective analyses of clinical histories of hospitalized or prospectively-monitored patients in specialized clinics or patients referred to specialists in hospitals. A select population is seen in these institutions, composed of the most serious cases and cases receiving the most comprehensive medical care, so information that they provide has selection biases that makes it difficult to extrapolate results to other sites, even within in the same country.[12,15]
Many published mortality studies are monocentric, where the cause of death is determined by reviewing clinical histories and autopsy reports, or by contacting the physician who cared for the patient in their final moments. Others use statistics from medical services, disability pensions from institutions and hospital statistics, which are not representative of the general population and are, therefore, often biased.[12,15]
SAD mortality studies conducted in Cuba are the result of case series that summarize the experience of a single doctor or institution (generally at secondary- or tertiary-care levels), with small samples, focused on a single disease. One of their limitations is that they do not capture mortality from SAD over time, nor do they reflect the true burden of these diseases within the general population. Studies that evaluate a single entity are useful in estimating its relevance as a health problem but tend to overestimate the importance of the specific condition on which they are focused.[12,15,42]
SAD mortality using multiple causes-of-death analysis The growing availability of data generated during medical care and monitoring of SAD incidence and clinical progression have transformed the outlook of health research. There was a time that health data collected through routine procedures was considered to be of no investigative interest. But these sources of information (including specific disease registries, primary care databases, administrative databases, archives, cancer registries, mandatory disease-reporting registries and mortality registries) open up opportunities for innovative, efficient, and cost-effective research to aid decisions made in clinical practice; to plan health services, programming and policies; and to improve patient care and healthcare efficiency in various settings and geographic regions worldwide.[43]
Mortality registries have been used as data sources to research mortality in SAD patients, [12,18,20–26] but there are few studies based on these registries despite the fact that they allow for closer examination of population mortality rates, and for inclusion of a greater number of patients over a longer period, which reduces selection bias and sample size problems. They are also more representative of the population than traditional cohort studies conducted among patients who receive specialized care.[15] Moreover, such prospective cohort studies are common for rheumatological research but require close patient monitoring and are expensive, which means that they are not always a viable alternative to research SAD in low-income environments.[9,15,31]
Despite Cuba’s high-quality mortality statistics,[33] only one study was conducted in Cuba on the mortality registry that included SAD among the immunopathological conditions considered,[44] which opens up an opportunity for research into a topic that has been understudied in the country.
Death is the result of complex pathological interactions, so the declared underlying cause of death may not entirely reflect causal processes. This aspect is important in SAD patients who often have combinations of diseases with physio-pathological implications and prognoses that are not entirely clear.[12,19] One methodological alternative to resolve this problem is to use multiple cause-of-death analysis. This term identifies an approach to mortality that considers all causes of death reported on the death certificate with no distinction between underlying and circumstantial causes.[18,19,28]
Analyzing multiple causes of death is a powerful epidemiological tool facilitating a more comprehensive understanding of the morbidity process.[18] Often the underlying cause of death is difficult to predict or is diagnosed after the fact. Conversely, the causes or complications derived from underlying causes may be predicted and even expected, which may help in monitoring or even prevention.[19,27]
While multiple cause-of-death analysis has been used since the first half of the 20th century, the last decade has seen an increase in epidemiological research that uses it as a strategy to address death from various health problems such as: opioid use,[45] obesity,[46] cardiovascular diseases,[47] aortic aneurysm dissection,[48] diabetes mellitus,[32] pulmonary embolism,[49] sepsis[50] and hemophilia.[51]
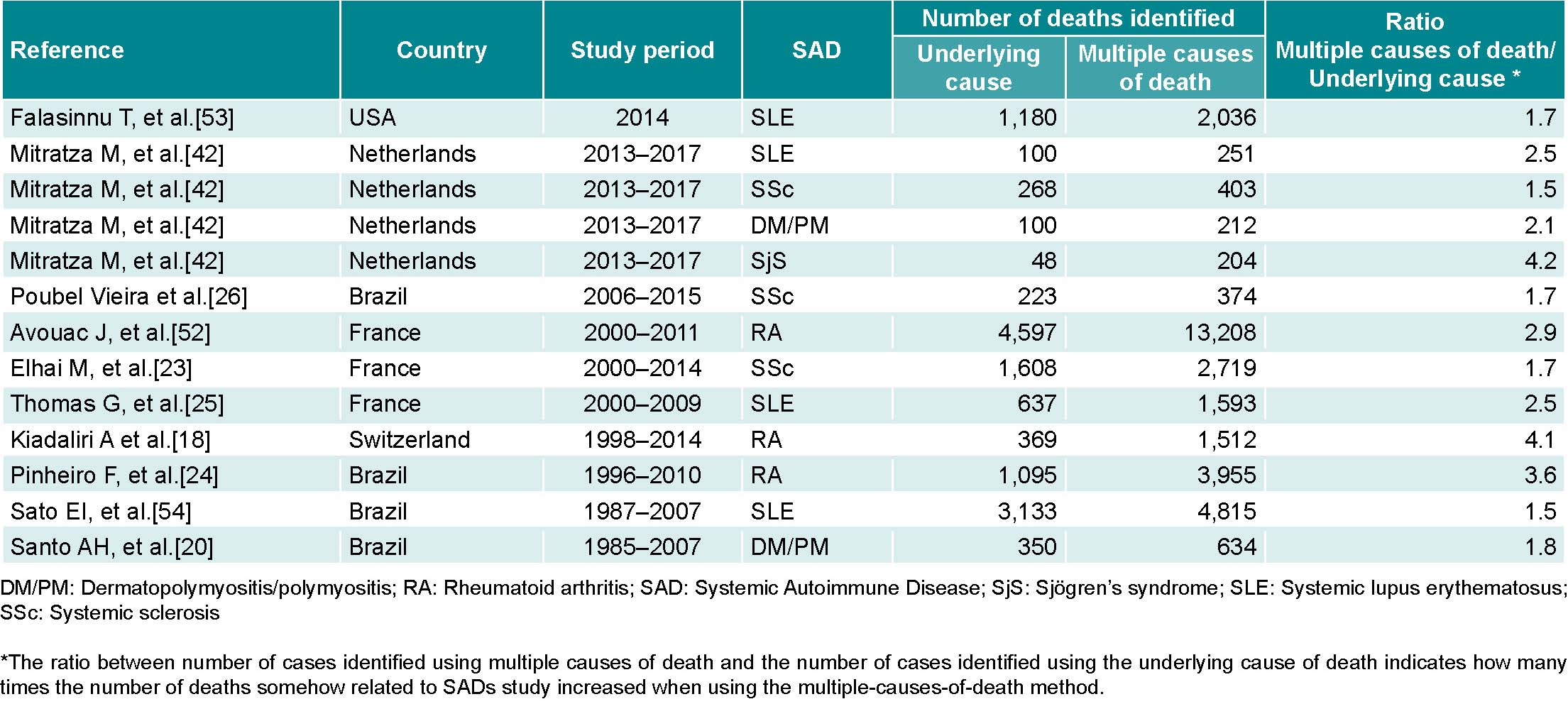
In countries with medium- or high-quality mortality registries representative of the population, these should be used as a source of SAD data, thus reducing patient selection bias and allowing for comparisons between countries as well as study of disease behavior over long periods at a relatively low cost. This potential is revealed in studies carried out in six countries, which show that deaths related to SAD that were identified using multiple-cause-of-death analysis increased 1.5 to 4.2 times compared to those identified using only the underlying cause (Table 1).