INTRODUCTION
Primary or inherited immunodeficiencies (PIDD) are infrequent. Their prevalence varies by type of genetic defect; while selective IgA deficiency is relatively common, more serious defects such as severe combined immunodeficiency are rare. New immunodeficiencies are continually being discovered, so the exact prevalence is unknown, although considered to be low.[1] In Cuba these diseases are underreported because of lack of specific diagnosis, among other causes.[2] The global incidence of IgA deficiency varies by ethnic origin. In the USA, estimated PIDD frequency varies between 1:1000 and 1:223 in the general population, and is much lower, between 1:18,000 and 1:2600 in those of Asian origin.[3]
According to criteria of the Pan American Immunodeficiency Group and the European Immunodeficiency Society, two clinical forms of IgA deficiency complete and partial deficits are included as PIDDs “predominantly due to antibody defect” and may be associated with failure in formation of antibodies against polysaccharide antigens or deficiencies of IgG2. When secretory IgA is lacking, patients may remain asymptomatic (70% of those affected) or develop florid clinical presentations in which infectious, allergic and autoimmune diseases predominate.[3,4]
IgA deficiency was the most frequent PIDD among patients with suspected immunodeficiencies seen at the Ciego de Ávila provincial Immunology Service during the six years of this study (1 of every 101). Thus, PIDD is considered an important health problem for immunology and allergology services in the province. Other PIDDs were less frequent, 1 of every 509 seen (administrative data, Immunology Service, Roberto Rodríguez Fernández Provincial General Teaching Hospital).
Recurrent bacterial respiratory infections are the most common health problem in patients with IgA deficiency; some can also develop gastrointestinal parasitic infections, such as giardiasis, and allergic and autoimmune disorders. An association between IgA deficiency and bronchial asthma is also reported.[3–6]
IgA deficiency has no specific or replacement treatment. Infections are treated with antimicrobials depending on the sensitivity of the causal agent. Some patients need prolonged antibiotic prophylaxis against infections to avoid complications. When allergic and autoimmune diseases coincide with IgA deficiency, specific treatment for each disease is used. A therapeutic alternative could be replacement or supply of external sources of IgA, but blood products that contain it (human immunoglobulin preparations) are not recommended, due to risk of anaphylactic reactions in patients with anti-IgA antibodies.[7,8] These treatment limitations led to a search for effective and noninvasive options to improve patients’ immune response, particularly against infections.
The normal immune response involves cellular receptors, costimulatory signals, cytokines and regulation by oxidation–reduction (redox) processes.[9] In recurrent infections, as well as allergic and autoimmune diseases, the balance between free oxygen radical production and antioxidant systems is tilted in favor of the former, causing oxidative damage to proteins, lipids, DNA, organs and systems. Reversing the effects of free radicals and regulating the redox state requires safe and effective therapeutic interventions; ozone has been suggested as an appropriate treatment.[10,11]
Ozone’s immune system effects have been described by Bocci and Larini.[10,12] Ozone is a prodrug with oxidizing properties, which produces biological effects in the body with a biphasic dose response (hormesis). Therapy is based on reversion of chronic oxidative stress at the cellular level and modulating effects on immune system function.[10]
In Cuba, ozone has had beneficial effects in management of various secondary humoral and cellular immune deficiencies, including HIV/AIDS, autoimmune diseases such as rheumatoid arthritis, and phagocytic immunodeficiencies.[13–15] Several studies show the immunomodulatory, antioxidant and anti-inflammatory effects of ozone therapy.[13–16]
IgA-deficient patients in Cuba may benefit from the demonstrated utility of ozone therapy for treatment of various conditions, its safety and well-understood main mechanisms, if used under internationally recommended good practice standards.[17] This therapy may successfully treat conditions caused by the underlying disorder and accompanying diseases, both infectious and due to immune system deregulation. We therefore proposed evaluating the potential benefits and adverse effects of ozone treatment in such patients.
METHODS
A monocentric, randomized, controlled, phase 2, open-label clinical trial (RPCEC 00000236) was carried out. Participation criteria were defined: inclusion (age 5–50 years, either sex, complete or partial IgA deficiency, selective or with deficient response of specific antibodies, isolated or associated with allergic or autoimmune diseases); exclusion (blood transfusions three months before or during the trial, any immunomodulatory treatment within the previous 6 months); and withdrawal (serious adverse reactions; treatment noncompliance or abandonment). Needed statistical power was calculated, considering prevalence variability and acceptable type 1 error.
The study population comprised 40 patients, aged 5–50 years, of both sexes, seen in the Immunology Service from January 18, 2010 through September 12, 2016. There were no withdrawals. Adults provided written informed consent to participate in the trial; parents gave written concent for participating children.
Patients were assigned by systematic random sampling to two groups, experimental (EG) and control (CG), 20 cases each. Members of both groups received specific treatment for their allergic disorder or autoimmune disease.
Treatment The EG received two cycles of ozone therapy by rectal insufflation. Each cycle consisted of 20 sessions (5 per week), with a 3-month interval between cycles. Ozone was produced by an ozone generator (OZOMED plus, Havana, Cuba). Recommended age-specific doses were applied according to the following sche-dule.[18]
- 5–10 years: 1.25–3 mg (25 mg/L concentration in 50 mL to 30 mg/L in 100 mL)
- 11–15 years: 2.25–4.2 mg (30 mg/L in 75 mL to 35 mg/L in 120 mL)
- >15–50 years: 2–8 mg (20 mg/L in 100 mL to 40 mg/L in 200 mL)
The CG received Hebertrans transference factor (Genetic Engineering and Biotechnology Center, Cuba). Dosage was one unit per m2 of body surface subcutaneously, once weekly for 12 weeks.[19]
Variables Therapeutic response was assessed as complete, partial, stable disease and progressive disease.
- Complete: satisfactory clinical status (absence of infectious diseases characteristic of these patients), increased IgM or IgG; normalization of all pro-oxidant parameters and increase of ≥2 antioxidant parameters
- Partial: satisfactory or acceptable clinical status (absence or decrease in severity and frequency of infectious diseases characteristic of these patients), increased IgM or IgG, normalization of ≥1 pro-oxidant parameter and increase of ≥1 antioxidant parameter
- Stable disease: presenting one or two of the following clinical status the same as at onset, no increased IgM or IgG, no normalization of pro-oxidant parameters, and no activation of antioxidant parameters
- Progressive disease: with three or four of the following worsening clinical condition, no increased IgM or IgG, no normalization of pro-oxidant parameters and no activation of antioxidant parameter
Serum was obtained by centrifugation and decantation after clot retraction, and simple radial immunodiffusion was used for immunoglobulin quantification, results expressed in mg/mL. Specific antibodies against tetanus and diphtheria toxoids were determined by ELISA, results expressed in IU/mL. Absolute values of lymphoid populations were estimated by flow cytometry.[20,21]
Plasma was obtained to assess redox state biomarkers, using EDTA as anticoagulant and erythrocyte lysate.
Pro-oxidation biomarkers: Concentration of malondialdehyde (MDA), a lipid peroxidation marker, was determined in plasma by the method described in LPO-586TM of the BIOXYTECH assay (OXIS Indague, USA), values expressed in µmol/L.[22] Advanced oxidation protein products (AOPP) were evaluated by Witko’s spectrophotometric technique, values expressed in µmol/L.[23] A colorimetric method was used for total peroxide quantification, based on oxidation of ferrous to ferric ions mediated by hydrogen peroxide (H2O2) under acidic conditions, results expressed in µmol/L.[24]
Biomarkers of antioxidant defense: Superoxide dismutase activity was determined by Marklund’s indirect kinetic method,[25] results expressed in percentage inhibition/minute/mL of enzyme (U/mL). Erythrocyte catalase activity was determined by a direct kinetic method using H2O2 as substrate, results expressed in mmol H2O2/t/min/mL.[26] Cellular glutathione peroxidase activity was determined by Paglia and Valentine’s technique, units of enzymatic activity expressed in mU/mL.[27] Plasma concentration of protein thiols, referred to as reduced glutathione, was determined by Sedlak and Lindsay’s technique, results expressed in µmol/L.[28] Total antioxidant capacity of plasma was measured by the ferric-reducing plasma ability assay, an indicator of antioxidant power and plasma ability to reduce ferric ions to ferrous, results expressed in mM Fe2+/L .[29]
Data collection and management Information was collected in patient registers and data collection notebooks.
Analysis Simple descriptive statistics demonstrated group homogeneity of demographic, clinical and immunological factors. The dependent-samples T test was used to compare groups with respect to response variables, comparing before and after treatment values in each group, and changes between groups by the double difference method. SPSS version 15.0 was used for analysis. The threshold specified for statistical significance was p ≤0.05.
Ethics Safety-related variables were analyzed as stipulated by the National Drug and Medical Equipment Quality Control Center (CECMED) in Regulation No. 45-2007 for notification and reporting of serious or unexpected adverse events in clinical trials.[30] Adult participants and children’s parents or legal guardians were given a detailed explanation of the trial, treatments used, and informed that they would receive one of two therapeutic options, both of which were expected to provide benefits by improving or temporarily eliminating some clinical manifestations of disease;[10,12–19] that allocation would be randomized, to avoid subjectivity; and that participants could abandon the trial at will, without prejudice to care. The trial was approved by the Research Ethics Committee of the Roberto Rodríguez Fernández Provincial General Teaching Hospital (main sponsor) and complied with the principles of the Helsinki Declaration.[31]
RESULTS
At trial onset, the groups were homogeneous with respect to demographic, clinical and immunological factors. Most patients were aged 5–10 years (14 cases in the EG and 16 in the CG) and male sex predominated (Table 1). Susceptibility to infection was evidenced by history of recurrent bacterial respiratory infections (3 events in one year and inadequate response to antibiotics) in 33 cases (18 in the EG and 15 in the CG) (82.5% of total) and by infectious parasitic diseases such as giardiasis in 17 patients (42.5% of total, 8 in the CG and 9 in the EG). Among concurrent diseases, allergy ranked first (32 cases, 80%) and of these, bronchial asthma was the most frequent with 25 cases (62.5% of total, 14 in the EG and 11 in the CG). Autoimmune diseases were diagnosed in 2 patients, one with rheumatoid arthritis (EG) and another with vitiligo (CG).
Immunological alterations were detected at trial onset, including decreased antibodies against protein antigens of tetanus and diphtheria toxoids in 2 patients, with values lower than those considered protective for these diseases (>0.1 IU/mL) and below-normal CD19+ B cell levels (>1%) in 10 cases. According to these results for immunological parameters, 95% (38/40) of cases showed selective IgA deficiency and 5% (2/40) IgA deficiency associated with functional response deficit (Table 1).
At treatment completion, complete IgA deficiency (IgA values below 0.07g/L) was found in 10 cases (25%) and partial (IgA values at least 2 standard deviations below normal values for age) in 30 (75%); IgM and IgG were normal in these cases at onset. After two cycles of ozone therapy, significant differences were observed between the EG and CG in IgG (p = 0.000) and IgM (p = 0.033) (Table 2).
The results of the oxidative stress study (Table 3), showed imbalances in the redox system with respect to normal reference values, since AOPP and MDA were elevated at study onset in both groups, indicating that patients with IgA deficiency had a pro-oxidant redox state. Reduced glutathione was elevated in 80% of patients and glutathione peroxidase was 60% below reference values for healthy individuals.
After two cycles of ozone therapy, AOPP (p = 0.003) and MDA (p = 0.001) decreased. Levels of total peroxides did not vary, and reduced glutathione was normalized (p = 0.032). Redox balance was favored in the EG, since significant increases were found in several antioxidant defense markers, such as superoxide dismutase (p = 0.001), cellular glutathione peroxidase (p = 0.012) and ferric reducing ability of plasma (p = 0.001) (Table 3). Increased antioxidants and decreased pro-oxidants were not found in the CG.
Table 1: Demographic and clinical characteristics of IgA-deficient individuals
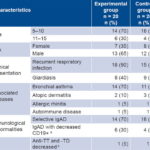
a normal >1% b normal >0.1 IU/mL CD19+: B-lymphocyte positive for CD19 antigen IgAD: IgA deficiency anti-TD: diphtheria toxoid antibody anti-TT: tetanus toxoid antibody
Table 2: Immunoglobulin levels in IgA-defi cient individuals treated with ozone or Hebertrans
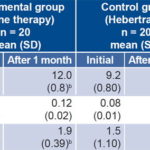
a associated with double difference b significant differences between values before and after treatment NS: not significant
Table 3: Pro-oxidant and antioxidant biomarkers in IgA-deficient individuals before and after treatment
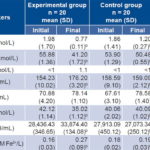
a associated with double difference b significant differences between values before and after treatment AOPP: advanced oxidation protein products CAT: catalase FOX: total peroxides FRAP: ferric reducing antioxidant power GSH: reduced glutathione GPx: glutathione peroxidase MDA: malondialdehyde SOD: superoxide dismutase NS: not significant
Therapeutic response was complete in 85% (17/20) of the EG, and partial in 15% (3/20). Complete response was found in 45% (9/20) of the CG, partial in 35% (7/20); disease was stable in 20% (4/20). Adverse events in the EG were mild and transient, and included tympanites immediately after rectal insufflation in two patients and abdominal pain in one. In the CG, erythema and pain at the injection site were reported by 8 patients (40%).
DISCUSSION
Some authors consider that IgA deficiency can be diagnosed in young children, since the majority of patients, who have IgA below 0.07 g/L at age 3 years (77%) are still IgA deficient at age 7 years.[4,8] However, since it could be confused with transient immunodeficiency of childhood, which occurs in young children due to delay in immune system development after birth,[3] we hold that age is an essential criterion for diagnosis, and that diagnosis of IgA deficiency should be made after the age of possible confusion with transient immunodeficiency of childhood; hence the lower limit in our study population.
The presence of recurrent respiratory and digestive infectious diseases is consistent with that reported by Domínguez in a chart review of 330 patients with IgA deficiency[32] and De Oliveira in a review of 39 patient files.[33] IgA deficiency manifests more frequently as digestive and respiratory system infections, as in the study cases, because IgA is predominant in mucosal secretions. IgA has an important biological function in mucous tissues, since it can neutralize viruses, bind to toxins, agglutinate bacteria, prevent bacteria from binding to epithelial cells and inhibit absorption of food antigens, thus preventing their entry into the bloodstream. These functions explain why IgA deficiency leads to a more aggressive bacterial microbiota and predisposes to local inflammatory processes. It has been suggested that low serum IgA levels cause less IgA transport to mucosal surfaces.[9] As in other studies, we found partial IgA deficiency was more frequent than complete deficiency.[32,33]
IgA deficiency is associated with a high prevalence of allergies, as found in our study, in which 80% of patients had clinical manifestations of these diseases. The literature reports that allergic disorders may be the initial clinical manifestation.[33,34] This may be because lack or insufficient concentrations of IgA causes partial loss of ability to block allergen entry through the mucosa, which induces sensitization and predisposes to development of allergies. Although the mechanism for association between bronchial asthma and IgA deficiency is unknown, some genetic defects, such as TNFRSF3B variants, may increase risk of this disease.[34]
Basal serum concentrations of specific antibodies prevaccination may be protective and are therefore valuable for studying humoral immunocompetence in IgA deficiency, since the humoral component of the immune response is compromised. The decreased B lymphocyte population in 10 patients is consistent with the findings of a Brazilian study.[35] Some studies show decreased CD4+ T lymphocytes in IgA-deficient patients.[36,37] Existence of an associated immune defect indicates that patients require more effective monitoring. It is important to study cell markers and activation in these patients, since IgA deficiency can progress to a common variable immunodeficiency.
Due to lack of specific treatment for IgA deficiency in Cuba, it is treated with Hebertrans because of its favorable clinical effects and immunomodulatory actions (expressed by an antigen-dependent and specific late cutaneous hypersensitivity reaction, in addition to other effects related to cell-mediated immunity).[38,39] Taking into account published evidence of these effects, Hebertrans was administered to the control group to comply with the ethical imperative of using the best available product comparable to the one being tested.
According to Viebahn-Hänsler, ozone requires systemic application to achieve good immunomodulation results. Rectal insufflation has been reported to have a systemic effect similar to major autohemotherapy in 90% of patients and it is the route of choice in children, older adults and patients who cannot tolerate intravenous therapies.[18] The biological effect of rectal ozone insufflation pathway has been demonstrated in preclinical models and clinical research.[40]
The significant increases in IgG and IgM after two cycles of ozone therapy show the effect of ozone on these immunological parameters, favorably impacting clinical status of patients who had good therapeutic response by decreasing infection recurrence. This result is based on the role of immunoglobulins in the humoral response of adaptive immunity; their functional integrity is essential to maintain immune system homeostasis. Various studies demonstrate the immunomodulatory effect of ozone therapy on antibody response and other immunological parameters.[15,41–43]
The fact that in this series of patients, AOPP and MDA were high in the initial assessment reveals the predominance of pro-oxidant damage. This is probably a consequence of intense and sustained activation of oxidative enzymes during recurrent infections and inflammatory processes, causing an excess of free radicals surpassing the capacity of endogenous antioxidant systems, with subsequent damage to proteins and lipids. The effect of free radicals on lipids is known as lipid peroxidation, and leads to destruction of the original lipid and loss of membrane integrity. MDA is a marker of this process. The effect on proteins causes amino acid oxidation, cross-linking of peptide chains and formation of carbonyl groups, evidenced by AOPP elevation.[44] From these results presence of inflammatory type oxidative stress can be inferred, caused by excessive activation of the natural mechanisms that generate reactive species and are associated with greater activation of the enzyme NADPH oxidase involved in chronic inflammation.[44]
The increase in pro-oxidant parameters and consequent oxidative stress is highly relevant to health–disease mechanisms, since it is associated with various pathological processes; in immunodeficiencies, it causes immune system dysfunction, which worsens existing deficits.[44–46] Immune system functioning is strongly influenced by redox balance, particularly in cells that have cytotoxic and phagocytic functions, which, due to their microbicidal activity are connected to free radical generation and deteriorate under oxidative stress. In IgA deficiency, other associated conditions, such as allergies and autoimmunity, worsen redox balance, resulting in tissue damage by free radicals. The pathophysiological repercussions of oxidation in IgA-deficient patients with allergies or autoimmunity are further immune dysfunction and lower response to immunomodulatory treatments.[15,47]
The significant increase in antioxidant parameters and decrease in pro-oxidants after two cycles of ozone therapy in the EG compared to the CG is a consequence of oxidative preconditioning, which ensures that a cycle of 20 ozone treatments is enough to maintain the positive effect for approximately 3 months, depending on the disease and patient response.[40]
The importance of this result is that the increase in antioxidant enzymes prevents free radicals from damaging vital structures and halts production of new oxidant molecules. As cells shift from an oxidizing to a reducing environment, they maintain membrain integrity and retain their specific functions, which, in the case of immune system cells, is defense against pathogenic microorganisms.[48]
The mechanism of ozone therapy’s antioxidant action at controlled doses is based on its ability to act as a hormetic stressor, which activates the related erythroid nuclear factor 2, Nrf2, in cytoplasm. When released from the adapter protein Keap1, this transcription factor binds to the DNA regions that regulate antioxidant response, increasing expression of genes involved in this defense. In an in vitro assay, Bocci identified several transcription factors in peripheral blood cells activated by ozone, such as: Nrf2, NFAT, AP-1 protein, and NF-Kappa B. Activation of Nrf2 could be a common denominator among cellular hormetic activators.[49] The importance of these transcription factors in the immune response is based on their participation in intracellular signaling pathways for activation of T lymphocytes,[9] whose adequate function is essential for defense against infection.
Ozone therapy is well tolerated and safe, with very few adverse events, since the rectal route is relatively noninvasive and painless and does not require use of needles or syringes.[40] This investigation shows similar safety and reactogenicity results to a 20-year followup study where ozone therapy was applied in patients with retinitis pigmentosa.[50] More intense and long-lasting adverse events were identified In the CG, such as pain at the injection site, but these were predominantly local and less severe than previously described for Hebertrans.[51] Distension and reddening are caused by local inflammation and recruitment of inflammatory cells after several applications.
The study’s monocentric nature is a limitation, since it prevents generalization of these findings as guidelines for other hospitals.
CONCLUSIONS
Our research results support ozone therapy as a suitable therapeutic option in treatment of IgA deficiency, because it produces antioxidant and immunomodulatory effects and is feasible, safe and minimally invasive.
ACKNOWLEDGMENTS
We thank Dr Maikel Roque Morgado, biostatistician for his support with data analysis.






