ABSTRACT
INTRODUCTION:Co-infections between hepatitis B and HIV viruses are frequent due to their similar epidemiological characteristics. Worldwide, hepatitis B infection is one of the main causes of hepatocellular carcinoma and cirrhosis. In Cuba as elsewhere, prevalences of hepatitis B and hepatitis C viral infections are higher in persons with HIV. These hepatitis viruses act as opportunistic infections in persons with HIV. In other contexts, persons with HIV have been found to be at higher risk for occult hepatitis B, defined as the presence in serum or plasma of hepatitis B virus DNA and antibodies to its core antigen, in the absence of hepatitis B surface antigen.
OBJECTIVES: Describe occult hepatitis B prevalence in Cuban HIV-positive patients and explore possible associations with their clinical characteristics.
METHODS:A total of 325 serum samples from patients positive for HIV and negative for hepatitis B surface antigen were studied, divided into two groups, Group 1, negative for hepatitis C virus; and Group 2, positive for hepatitis C virus. Exposure to hepatitis B was determined by testing for hepatitis B core antigen; samples positive for hepatitis B core antigen were then examined for presence of antibodies to hepatitis B surface antigen. Both determinations were done by ultramicroELISA. In samples positive for hepatitis B core antigen with levels of antibodies to hepatitis B surface antigen of <50 IU/L, real-time polymerase chain reaction was used to detect hepatitis B DNA and its presence examined in relation to several clinical variables. All data were obtained from patients’ clinical records.
RESULTS: In the hepatitis-C–negative group, 27.9% (68/243) of serum samples tested were positive for hepatitis B core antigen. In the hepatitis-C–positive group, 37.8% (31/82) were positive for hepatitis B core antigen. Total hepatitis B virus exposure prevalence was 30.4% (99/325); 54.5% (54/99) showing low immunity (hepatitis B virus surface antigen <50 IU/L) and 24% of these (13/54), occult hepatitis. There was no statistically significant association between hepatitis B virus DNA and any of the clinical variables studied.
CONCLUSIONS: Low-immunity HIV-positive persons in our study were exposed to hepatitis B virus. Diagnosis of occult hepatitis B infection is frequent in these patients. This study suggests that diagnostic protocols for persons with HIV and without hepatitis B surface antigen should include testing for hepatitis B core antigen, with positive results followed by molecular techniques to detect occult hepatitis B. This study makes a useful contribution to prevention and control of hepatitis B in Cuba.
KEYWORDS: Hepatitis B, hepatitis B antigens, hepatitis C, AIDS, AIDS-related opportunistic infections, Cuba
INTRODUCTION
Co-infections with hepatitis B (HBV) and HIV viruses are a public health challenge, due to their increasing incidence, population impact and similar epidemiological characteristics.[1] HIV affects the prognosis and clinical course of HBV and thus prospects for its prevention and control. HBV is considered an opportunistic infection in HIV patients, when diminished immunity accelerates the progress of hepatitis B virus (HBV) infection to chronic hepatitis.[2–4] HBV prevalence is higher in HIV-infected individuals than in the general population; in some regions of the world, one of every three HIV-infected individuals has HBV or hepatitis C (HCV) markers, or both.[5]
Traditionally, HBV is diagnosed by serology techniques to detect antigens or antibodies. Since the 1990s, in Cuba and elsewhere, molecular techniques have enabled detection of HBV DNA.[6,7] HBV surface antigen (HBsAg) in serum or plasma is the marker commonly used to diagnose HBV infection, but there are persons in whom HBV DNA is detected in the absence of HBsAg.[8,9] Such occult HB infection has been found in patients:[9]
- with HBV risk factors;
- with hepatic carcinoma;
- who are chronic HBV carriers;
- with HBV–HCV co-infection;
- who are immunosuppressed;
- with inexplicable increases in hepatic enzymes (cryptogenic cirrhosis);
- who are co-infected with HIV and HCV.
Occult HBV is frequent in persons with HIV, its detection depending on the molecular techniques used and the endemic pattern of both viruses in the populations studied.[1] It is also reported to be very frequent in persons with HIV−HCV co-infection. Immunosuppressed HIV-positive persons may not respond to recombinant HBV vaccine or have low antibody response to HBsAg.[1,2] Hence, in HIV patients who test HBsAg negative, HBV DNA should be determined before starting high activity antiretroviral therapy (HAART) so that anti-HBV antiretrovirals can be included if necessary.
At the end of 2009, there were 12,217 HIV-positive persons in Cuba,[10] but occult HBV studies among these individuals have not been carried out. Research on such patients with HBsAg negative sera would have important implications for appropriate clinical management and improved prognosis.
The objectives of this study were to describe occult HBV prevalence in HIV-positive persons in Cuba and to explore possible associations between occult HBV infection and patients’ clinical characteristics.
METHODS
A cross-sectional study was carried out using 325 HBsAg-negative serum samples from among the 1770 HIV-positive adults treated at the Pedro Kourí Tropical Medicine Institute (IPK, its Spanish acronym) during 2008 and 2009. At IPK, HBsAg and HCV antibody (anti-HCV) tests are included in annual follow up of HIV-positive persons because of the frequent association between HIV and these two hepatitis viruses. The HBsAg-negative samples were divided into two groups: anti-HCV–positive and anti-HCV–negative. HBV exposure was determined by presence of antibodies to HBV core antigen (anti-HBc); anti-HBc–negative sera were excluded from the study. Immunity was assessed by detection of antibodies to HBV surface antigen (anti-HBs). Anti-HBc–positive sera with anti-HBs levels of <50 IU/L were examined for viral load by HBV DNA detection. The diagnostic algorithm is displayed in Figure 1.
Figure 1: Diagnostic algorithm

CR: clinical record
A form was prepared to record variables from clinical records: time elapsed from HIV diagnosis, AIDS stage, alanine aminotransferase (ALT), aspartate aminotransferase (AST), erythrosedimentation rate and CD4 cell count. Not all data were available for all cases, so totals are not uniform across variables.
Serological techniques UltramicroELISA (enzyme-linked immunosorbent assay) was used to test all serum samples for anti-HBc, to determine HBV exposure. Samples with fluorescent values equal to or below the threshold value (0.2 x median of the negative controls) were considered positive. All samples positive for anti-HBc were tested for anti-HBs, also with ultramicroELISA. Anti-HBs levels of ≥10 IU/L were considered protective; levels <10 IU/L were considered non-protective or negative.
Traditionally, the degree of immune response is classified thus:[11]
- Non-protective: anti-HBs <10 IU/L
- Hypo response: anti-HBs 10 IU–99.9 IU/L
- Normal response: anti-HBs 100 IU–999.9 IU/L
- Hyper response: anti-HBs >1000 IU/L
In this study, low immunity was defined by anti-HBs levels of <50 IU/L, and a normal or good response by levels of ≥50 IU/L, as recommended by several authors.[1,9]
Diagnostic kits (UMELISA Anti-HBsAg, UMELISA Anti-HBcAg, UMELISA HBsAg) used were produced by the Immunoassay Center, Havana, Cuba. For both techniques the MW2001 washer (TecnoSuma, Cuba) and the PR-521 reader, (TecnoSuma, Cuba) were used. Test validity and results interpretation were performed automatically by the manufacturer’s program (software package for strip readers, version 8.0).
Molecular techniques Real-time polymerase chain reaction (RT-PCR) was used to detect and quantify HBV DNA in samples that had exhibited exposure to HBV and low immunity to it (anti-HBc−positive and anti-HBs <50 IU/L). The technique was standardized following the protocol described by Chen et al.[12] with slight modifications, using a probe kit amplifying a 120 bp region of the HBV genome core. An in-house serum standard was used to construct the calibration curve, enabling DNA quantification.
DNA extraction from serum samples using proteinase K Eighty µL of serum were used, adding 20 µLlysis buffer [20 mM Tris-HCl (pH = 8.0), 150 mM NaCl, 10 mM EDTA and 2% SDS] and 1 µL proteinase K (20 mg/mL) were added. After mixing, they were incubated at 56 °C for 1 hour and then at 100 °C for 10 min, to inactivate proteinase K. The mixture was then centrifuged at 8000 rpm (Eppendorf 5417R centrifuge, Germany) for 3 minutes and the clear aqueous supernatant transferred to a new tube.
Amplification, detection and quantification The commercial kit LightCycler TaqMan Master Mix (Roche, Germany) was used, consisting of a ready-to-use reaction mixture including: FastStar Taq DNA polymerase, reaction buffer, MgCl2, dideoxynucleotide mixture and nuclease-free water. Primers and probe were added to the mixture at 0.7 µM and 0.15 µM concentrations, respectively. Finally, 5 µL of control and sample DNA were added for a final volume of 20 µL. Amplification was carried out in 20 µL capillaries (LightCycler Capillaries, Roche, Germany) in a LightCycler 1.5 (Roche, Germany) with the following program:
- one 10-minute cycle at 95 °C (allows activation of Taq polymerase and denaturing of nucleic acids);
- 45 cycles of: denaturation at 95 °C for 15 sec, hybridization at 58 °C for one minute, and an extension at 72 °C for one second.
Analysis of results was performed automatically by the program at the end of the sample run. Results were considered positive from one copy forward, and nondetectable when there was no genome amplification.
Statistical analysis A database was prepared and processed using SPSS 11.5 and EpiInfo version 6.0. Absolute and relative frequencies, anti-HBc prevalence and HBV DNA presence were calculated. HBV DNA positivity was determined; univariate analysis performed, and prevalence ratio (PR) calculated as a measure of association, with 95% significance intervals. PR values greater than 1.5 were considered clinically significant. Socio-demographic and clinical variables were evaluated for samples that tested positive for anti-HBc and had anti-HBs levels of <50 IU/L (exposed to HBV with low immunity).
Ethical aspects The IPK Ethics Committee approved this study, including prior written consent by patients seen in clinical services, to allow performance of tests for additional viral markers to detect occult HBV infection. Results were stored in a password-protected database accessible only to researchers from the laboratory. Data obtained were used only in this study and for patient well-being.
RESULTS
Exposure to HBV and presence of immunity (anti-HBs) In Group 1, 28% (68/243) of samples were anti-HBc positive, compared to 37.8% (31/82) in Group 2. Thus, global exposure prevalence as measured by anti-HBc was 30.5% (99/325) (Table 1).
Table 1: Prevalence of HBV exposure (anti-HBc) in serum samples of HIV-positive/HBsAg-negative patients (n=325)
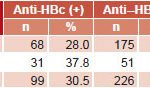
Source: National Reference Laboratory for Viral Hepatitis, IPK
HBV immunity levels were determined in positive anti-HBc serum samples (Table 2). Of 99 samples analyzed, 38 (38.4%) did not show protective titers of anti-HBs (<10 IU/L); 16 (16.1%) had low immunity (10–49.9 IU/L) and the remaining 45 (45.5%) had levels of ≥50 IU/L. The overall prevalence of HBV seroprotection (low or complete) was 61.6%.
Protective anti-HBs titers were more frequent in sera in the anti-HCV–negative group: 63.2% (43/68); the frequency of titers ≥50 IU/L was also higher in anti-HCV–negative group (50% vs. 35.5%) (Table 2).
Table 2: HBV exposure (anti-HBc+) by anti-HBs titers and presence of anti-HCV in study samples
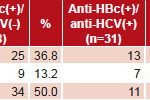
Source: National Reference Laboratory for Viral Hepatitis, IPK
HBV DNA detection and quantification When the 54 anti–HBc-positive sera with anti-HBs levels of <50 IU/L were tested for HBV DNA, HBV genetic material was identified in 24.1% (13/54) (Table 3). The mean number of HBV copies detected by RT-PCR was 10.5/5 μL DNA, ranging between 1 and 47 copies/5 μL.
HBV DNA was found in 28.9% (11/38) of sera exposed to HBV (anti-HBc–positive) without protective titers (anti-HBs levels of <10 IU/L). In exposed individuals with low immunity (anti-HBs levels of 10–49.9 IU/L), 12.5% (2/16) of serum samples were viremic (Table 4).
In anti-HCV–positive individuals, HBV genetic material was detected in 9.5% (2/21), in the absence of protective titers (anti-HBs <10 IU/L). In contrast, HBV DNA was identified in 33.3% (11/33) of anti-HCV–negative serum samples, predominantly in those with anti-HBs levels of <10 IU/L (Table 4).
Relation between presence of occult HBV and clinical variables There was no statistically-significant association between occult HBV infection and any of the clinical variables studied, although prevalence ratio point estimates exceeded 1.0 for some variables.
Table 3: HBV DNA detection by anti-HCV presence and anti-HBs titers (n=54)
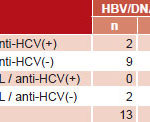
Source: National Reference Laboratory for Viral Hepatitis, IPK
Table 4: HBV DNA positivity by study variables in HBV-exposed HIV-positive sera with low immunity
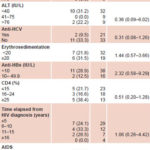
PR: Prevalence ratio CI: Confidence interval
DISCUSSION
HBV exposure prevalence observed was lower than the 45.5% anti-HBc prevalence reported by Rodríguez et al. in an earlier study of Cuban HIV-positive patients,[13] but this prevalence was in all patients, irrespective of HBsAg status. The lower prevalence observed in our study is not surprising; Cuba’s strategy of immunizing at-risk groups and the population aged <27 years has reduced morbidity from HBV.[14] A Cuban study of hemodialysis patients at risk for HBV exposure found a higher anti-HBc prevalence than we detected (50.3% vs. 30.4%).[15] Research by Ballester et al. to determine anti-HBc prevalence in at-risk groups in Cuba found prevalences of 52.5% in persons on hemodialysis, 56.8% in hemophiliacs, 34.7% in persons with malignant hemopathies, and 40.5% in persons with sickle cell disease.[16].
In HBsAg-negative persons with HIV in Brazil, anti-HBc positivity was higher than in this study (56% vs. 30.4%).[17] In Senegal, which has a high prevalence of HBV, the presence of anti-HBc in the general population was similar to that in persons with HIV (66.6%),[18] and notably higher than that obtained in this study. At the same time, relatively low prevalence in studies similar to ours were reported in Argentina (12.1%), South Africa (10.6%) and Spain (6.6%).[19–21]
Nebbia et al. had similar results to those reported in this study (39%) in HBsAg-positive persons with HIV in the United Kingdom.[22] Similarly, 35.5% anti-HBc prevalence was found in a comparable population studied in Italy.[23] Sucupira et al. reported 30.0% anti-HBc positivity in HIV patients in Brazil, similar to our findings.[24]
Licourt et al., found 5.6% anti-HBc reactivity in a preliminary analysis carried out in blood banks in low-HBV prevalence areas of Cuba,[25] in contrast with the 30.4% reported in this study of persons with HIV, supporting the notion that this group is at risk for HBV exposure.
Laguno et al. stratified anti-HBc by anti-HCV levels, and detected higher values than ours for HIV co-infected patients (60% vs. 37.8%).[26] Results similar to ours were reported by Arababadi et al. in a comparable population in Iran (33%).[27]
Our observation of higher anti-HBc levels in anti-HCV–positive individuals than in anti-HCV–negative persons has also been reported in blood donors.[28] This may be explained by the viruses’ shared transmission routes, independent of population group.
In Spain, a study similar to ours found a stronger association between the HBV exposure marker (anti-HBc) and anti-HCV (80.1%) than we did.[29] It is well known that the main transmission route for HBV and HCV in hemodialysis patients is parenteral. In the case of HIV, the parenteral route is pertinent mainly in persons who use intravenous drugs, a population with a high prevalence of HCV infection and in which often the only HBV marker found is anti-HBc.[30]
In Cuba, HIV transmission is predominantly sexual, which is relatively inefficient for transmitting HCV.[31] Our results reinforce the observation that frequency of HBV and HCV exposure markers in persons with HIV is likely related to their shared transmission routes.
Hemodialysis patients are another immunosuppressed population with increased risk of exposure to HBV; in hemodialysis patients with HBV exposure markers in Cuba, 91.2% have protective levels of anti-HBs. This higher level of seroprotection could be explained by Cuba’s HBV immunization schedule for persons on hemodialysis: given their immunosuppression, they receive annual boosters to prevent anti-HBs titers declining to non-protective levels.
Several researchers have reported lower rates of dual anti-HBc and anti-HBs reactivity than ours in HIV patients, ranging from 11.9% to 34.1%.[33–35] This could be related to the Cuban HBV vaccine (Heberbiovac) schedule, which calls for additional vaccinations if HIV-positive status is detected.[13]
Levels of anti-HBs ≥10 IU/L detection were found slightly more frequently in anti-HCV–negative than in anti-HCV–positive patients (63.2% vs. 58%). In a US study of persons with HIV, some of whom were co-infected with HCV, French et al. detected low anti-HBs titers, which could suggest influence of viral co-infection on protective levels or duration of anti-HBs.[36]
Declining anti-HBs titers have already been reported in persons with HIV after a standard vaccination schedule; which is why boosters are recommended,[37,38] suggesting that the immunocompromised status of HIV-positive persons influences loss of HBV immunity; that is, anti-HBs titers decline to non-detectable levels, a considerably different picture than that of immunocompetent persons. So, for example, the prevalence of protective levels of anti-HBs in anti–HBc-positive blood donors was 85.7%.[39]
Our observed occult HBV prevalence of 24.1% is within the range of values reported in the international literature.[22] Lukhwareni et al. reported a lower value (7.4%) in HIV patients under highly active antiretroviral therapy.[1]
Occult HBV has frequently been reported in HIV patients without anti-HBs protective levels.[40] The presence of HBV DNA in HIV-positive individuals with low or absent immunity to HBV was also reported by Nebbia et al., who consider lack of HBV immunity in HBsAg-negative persons who are anti-HBc positive a marker of occult HBV.[22] Lower values than in this study were detected in South African HIV patients on retroviral therapy (5.4%).[1] Generally, viral loads detected in occult HBV are low (<103 copies/mL), which is consistent with the present study.[1,41,42]
Occult HBV infection was identified in 33.3% (11/33) of anti-HCV–negative patients in this study, with levels of anti-HBs of <10 IU/L predominating. Other authors report higher frequencies of occult HBV in HIV patients co-infected with HCV.[5,26]
We did not find an association between occult HBV and elevated ALT or AST. Similarly, Lo Re et al. carried out a study in HIV individuals with and without occult HBV and did not find significant differences in transaminase levels.[43]
Elevated erythrocyte sedimentation rates are frequent in HIV-positive individuals, since they may have different infectious and non-infectious conditions that can lead to erythrocyte sedimentation rates of up to three figures.[44] We encountered no difference in this respect between people with and without occult Hepatitis B infection.
Contrary to reports in international literature,[5] we found HBV DNA more frequently in anti-HCV–negative sera than in positive. This could be explained by HCV replication interfering with HBV replication, reducing HBV load to levels undetectable with the techniques used.[26]
This study suggests a possible inverse association between level of anti-HBs and occult hepatitis B, but because of the cross-sectional design, it is impossible to determine which came first, so we cannot infer a causal relationship. Other authors have found this association; some even hold that HBV molecular diagnosis should be considered when anti-HBs is absent in sera from persons positive for both HIV and anti-HBc, and even more so in those lacking all HBV markers.[1,29,45]
Like Fabris et al.,[5] we found no association between occult HBV infection and CD4 cell count, time elapsed from HIV diagnosis and AIDS stage. Similarly, a study in Mexico detected no correlation between occult HBV and AIDS status.[46]
Besides its cross-sectional design, a further limitation of our study is that we could not identify the presence of active HVC infection by PCR in persons positive for both HIV and anti-HCV, which would allow determination of its effect on HBV replication and occult HBV detection.
CONCLUSIONS
Occult HBV diagnosis is frequent in HIV patients with poor immune response.
We recommend that the diagnostic algorithm for persons with HIV who are HBsAg-negative include anti-HBc detection and, if positive, follow-up with molecular diagnosis to detect occult hepatitis B. Such a diagnostic strategy could help reduce sequelae and HBV transmission in this population, thus improving their quality of life. It is feasible in Cuba, which has a domestically-manufactured diagnostic kit, enabling anti-HBc and anti-HBs testing for those requiring it.
This is the first study of its type in Cuba. Its results contribute to understanding hepatitis B in persons with HIV, which should help improve treatment, prognosis, control and prevention of this disease, as well as contribute to the well-being of those affected.
References

- Lukhwareni A, Burnett RJ, Selabe SG, Mzileni MO, Mphahlele MJ. Increased detection of HBV DNA in HBsAg-positive and HBsAg-negative South African HIV/AIDS patients enrolling for highly active antiretroviral therapy at a Tertiary Hospital. J Med Virol. 2009 Mar;81(3):406–12.
- Burnett RJ, François G, Kew MC, Leroux-Roels G, Meheus A, Hoosen AA, et al. Hepatitis B virus and human immunodeficiency virus co-infection in Sub-Saharan African: A call for further investigation. Liver Int. 2005 Apr;25(2):201–13.
- Modi AA, Feld JJ. Viral hepatitis and HIV in Africa. AIDS Rev. 2007 Jan–Mar;9(1):25–39.
- Mphahlele MJ. Impact of HIV co-infection on hepatits B prevention and control: A view from sub-Saharan Africa. SAJEI. 2008;23(1):14–8.
- Fabris P, Biasin MR, Giordani MT, Berardo L, Menini V, Carlotto A, et al. Impact of occult HBV infection in HIV/HCV co-infected patients: HBV-DNA detection in liver specimens and in serum samples. Curr HIV Res. 2008 Mar;6(2):173–9.
- Yokosuka O, Omata M, Hosoda K, Tada M, Ehata T, Ohto M. Detection and direct sequencing of hepatitis B virus genome by DNA amplification method. Gastroenterology. 1991 Jan;100(1):175–81.
- Galbán E, Vega H, Gra B, Rodríguez A, Doval MA, Haedo D, et al. Papel de la Ribavirina en el tratamiento de la hepatitis crónica B. Gastroenterol Hepatol. 2000 Apr;23(4):165–9. Spanish.
- Gutiérrez C, León G, Liprandi F, Pujol FH. Low impact of silent hepatitis B virus infection on the incidence of post-transfusion hepatitis in Venezuela. Rev Panam Salud Publica. 2001 Dec;10(6):382–7.
- Torbenson M, Thomas DL. Occult hepatitis B infection. Lancet Infect Dis. 2002 Aug;2(8):479–86.
- Estruch L, Santín M, Lantero MI, Ochoa R, Joanes J, Alé K, et al. República de Cuba. Informe nacional sobre los progresos realizados en la aplicación del UNGASS. La Habana [Internet]. Geneva: UNAIDS; 2010 Mar [cited 2010 June 20]. 42 p. Available from: http://data.unaids.org/pub/Report/2010/cuba_2010_country_progress_report_es.pdf. Spanish.
- André FE. Overiew of a 5 year clinical experience with a yeast-derived hepatitis B vaccine. Vaccine. 1990 Mar;8 Suppl:S74–8.
- Chen RW, Piiparinen H, Seppänen M, Koskela P, Sarna S, Lappalainen M. Real-Time PCR for Detection and Quantitation of Hepatitis B Virus DNA. J Med Virol. 2001 Oct;65(2):250–6.
- Rodriguez L, Collado F, Aragón U, Díaz B, Rivero J. Hepatitis B Virus exposure in Human Immunodeficiency Virus seropositive Cuban patients. Mem Inst Oswaldo Cruz. 2000 Mar–Apr;95(2):243–5.
- Delgado G. Situación de la Hepatitis B en Cuba. Actas del Taller Nacional de Hepatitis; 2008 Jun; Havana. Havana: Ministry of Public Health (CU); 2009. Spanish.
- Montalvo MC, Delgado G, Díaz M, Rodríguez L. Prevalence of hepatitis B virus markers and risk factors associated in haemodialysis patients from Havana City; 2002-2003. Nefrologia. 2007;27(2):234–5.
- Ballester JM, Rivero RA, Villaescusa R, Merlín JC, Arce AA, Castillo D, et al. Hepatitis C virus antibodies and other markers of blood-transfusion–transmitted infection in multi-transfused Cuban patients. J Clin Virol. 2005 Dec;34 Suppl 2:S39–46.
- Santos EA, Yoshida CF, Rolla VC, Mendes JM, Vieira IF, Arabe J, et al. Frequent occult hepatitis B virus infection in patients infected with human immunodeficiency virus type 1. Eur J Clin Microbiol Infect Dis. 2003 Feb;22(2):92–8.
- Diop-Ndiaye H, Touré-Kane C, Etard JF, Lô G, Diaw P, Ngom-Gueye NF, et al. Hepatitis B, C seroprevalence and delta viruses in HIV-1 Senegalese patients at HAART initiation (retrospective study). J Med Virol. 2008 Aug;80(8):1332–6.
- Quarleri J, Moretti F, Bouzas MB, Laufer N, Carrillo MG, Giuliano SF, et al. Hepatitis B virus genotype distribution and its lamivudine-resistant mutants in HIV-coinfected patients with chronic and occult hepatitis B. AIDS Res Hum Retroviruses. 2007 Apr;23(4):525–31.
- Firnhaber C, Viana R, Reyneke A, Schultze D, Malope B, Maskew M, et al. Occult hepatitis B virus infection in patients with isolated core antibody and HIV co-infection in an urban clinic in Johannesburg, South Africa. Int J Infect Dis. 2009 Jul;13(4): 488–92.
- Palacios R, Mata R, Hidalgo A, Muñoz L, Viciana I, Del Arco A, et al. Very low prevalence and no clinical significance of occult hepatitis B in a cohort of HIV-infected patients with isolated anti-HBc seropositivity: the BHOI study. HIV Clin Trials. 2008 Sep–Oct;9(5):337–40.
- Nebbia G, Garcia-Diaz A, Ayliffe U, Smith C, Dervisevic S, Johnson M, et al. Predictors and kinetics of occult hepatitis B virus infection in HIV-infected persons. J Med Virol. 2007 Oct;79(10):1464–71.
- Filippini P, Coppola N, Pisapia R, Scolastico C, Marrocco C, Zaccariello A, et al. Impact of occult hepatitis B virus infection in HIV patients naive for antiretroviral therapy. AIDS. 2006 Jun 12;20(9):1253–60.
- Sucupira MV, Mello FC, Santos EA, Niel C, Rolla VC, Arabe J, et al. Patterns of hepatitis B virus infection in Brazilian human immunodeficiency virus infected patients: high prevalence of occult infection and low frequency of lamivudine resistant mutations. Mem Inst Oswaldo Cruz. 2006 Sep;101(6):655–60.
- Bello, Marité (Institute of Tropical Medicine, Havana, Cuba). Conversation with: Licourt Tania, Delgado Graciela, Suárez Angel, Lefrán María Elena, Fonte Maira (Immunoesssay Center, Havana, Cuba). 2009 April 24.
- Laguno M, Larrousse M, Blanco JL, León A, Milinkovic A, Martínez-Rebozler M, et al. Prevalence and clinical relevance of occult hepatitis B in the fibrosis progression and antiviral response to INF therapy in HIV-HCV–coinfected patients. AIDS Res Hum Retroviruses. 2008 Apr;24(4):547–53.
- Arababadi MK, Hassanshahi G, Yousefi H, Zarandi ER, Moradi M, Mahmoodi M. No detected hepatitis B virus-DNA in thalassemic patients infected by hepatitis C virus in Kerman province of Iran. Pak J Biol Sci. 2008 Jul 1;11(13):1738–41.
- Bhatti FA, Ullah Z, Salamat N, Ayub M, Ghani E. Anti-hepatitis B core antigen testing, viral markers, and occult hepatitis B virus infection in Pakistani blood donors: implications for transfusion practice. Transfusion. 2007 Jan;47(1):74–9.
- Pérez-Rodríguez MT, Sopeña B, Crespo M, Rivera A, González del Blanco T, Ocampo A, et al. Clinical significance of “anti-HBc” in human immunodeficiency virus-positive patients. World J Gastroenterol. 2009 Mar 14;15(10):1237–41.
- Gandhi RT, Wurcel A, Lee H, McGovern B, Boczanowski M, Gerwin R, et al. Isolated antibody to hepatitis B core antigen in human immunodeficiency virus type-1–infected individuals. Clin Infect Dis. 2003 Jun 15;36(12):1602–5.
- Ministry of Public Health (CU). Informe Nacional sobre los progresos realizados en la aplicación del UNGASS, Cuba, 2008 [Internet]. Geneva: UNAIDS; 2010 Mar [cited 2010 Jun 20]. 39 p. Available from: http://data.unaids.org/pub/Report/2008/cuba_2008_country_progress_report_sp_es.pdf. Spanish.
- Montalvo Villalba MC. Perfil seroepdemiológico del virus de la hepatitis B en pacientes hemodializados de Ciudad Habana [master’s thesis]. Havana: Pedro Kourí Tropical Medicine Institute. (CU); 2004. 67 p. Spanish.
- Neau D, Winnock M, Galpérine T, Jouvencel AC, Castéra L, Legrand E, et al. Isolated antibodies against the core antigen of hepatitis B virus in HIV-infected patients. HIV Med. 2004 May;5(3):171–3.
- Rodríguez-Torres M, González-García J, Bräu N, Solá R, Moreno S, Rockstroh J, et al. Occult hepatitis B virus infection in the setting of hepatitis C virus (HCV) and human immunodeficiency virus (HIV) co-infection: clinically relevant or a diagnostic problem? J Med Virol. 2007 Jun;79(6):694–700.
- Ramia S, Mokhbat J, Ramlawi F, El-Zaatari M. Occult hepatitis B virus infection in HIV-infected Lebanese patients with isolated antibodies to hepatitis B core antigen. Int J STD AIDS. 2008 Mar;19(3):197–9.
- French AL, Operskalski E, Peters M, Strickler HD, Tien PC, Sharp GB, et al. Isolated hepatitis B core antibody is associated with HIV and ongoing but not resolved hepatitis C virus infection in a cohort of US women. J Infect Dis. 2007 May 15;195(10):1437–42.
- Rey D, Krantz V, Partisani M, Schmitt MP, Meyer P, Libbrecht E, et al. Increasing the number of hepatitis vaccine injections augments anti-HBs response rate in HIV- infected patients. Effect on HIV-1 viral load. Vaccine. 2000 Jan 18;18(13):1161–5.
- Cruciani M, Mengoli C, Serpelloni G, Lanza A, Gomma M, Nardi S, et al. Serologic response to hepatitis B vaccine with high dose and increasing number of injections in HIV infected adult patients, Italy. Vaccine. 2009 Jan 1;27(1):17–22.
- Kupski C, Träsel FR, Mazzoleni F, Winckler MA, Bender AL, Machado DC, et al. Serologic and molecular profile of anti–HBc-positive blood bank donors in an area of low endemicity for HBV. Dig Dis Sci. 2008 May;53(5):1370–4.
- Piroth L, Lafon ME, Binquet C, Bertillon P, Gervais A, Lootvoet E, et al. Occult hepatitis B in HIV-HCV coinfected patients. Scand J Infect Dis. 2008;40(10):835–9.
- Shire NJ, Rouster SD, Rajicic N, Sherman KE. Occult hepatitis B in HIV-infected patients. J Acquir Immune Defic Syndr. 2004 Jul 1;36(3):869–75.
- Tsui JI, French AL, Seaberg EC, Augenbraun M, Nowicki M, Peters M, et al. Prevalence and long-term effects of occult hepatitis B virus infection in HIV-infected women. Clin Infect Dis. 2007 Sep 15;45(6):736–40.
- Lo Re V 3rd, Wertheimer B, Localio AR, Kostman JR, Dockter J, Linnen JM, et al. Incidence of transaminitis among HIV-infected patients with occult hepatitis B. J Clin Virol. 2008 Sep;43(1):32–6.
- Pérez J, Pérez CD, González I, Díaz-Jidy M, Millán JC, Orta M. Pautas Cubanas para el tratamiento antirretroviral en los pacientes con VIH/SIDA. Havana: Ministry of Public Health (CU); 2004. 64 p. Spanish.
- Allain JP. Occult hepatitis B virus infection. Transfus Clin Biol. 2004 Feb;11(1):18–25.
- Torres-Baranda R, Bastidas-Ramírez BE, Maldonado-González M, Sánchez-Orozco LV, Vázquez-Vals E, Rodríguez-Noriega E, et al. Occult hepatitis B in Mexican patients with HIV, an analysis using nested polymerase chain reaction. Ann Hepatol. 2006 Jan–Mar;5(1):34–40.
THE AUTHORS
Marité Bello Corredor (Corresponding author: marite@ipk.sld.cu), microbiologist with a master’s degree in virology. Instructor and associate researcher, Pedro Kourí Tropical Medicine Institute, Havana, Cuba.
María Caridad Montalvo Villalba, physician with a master’s degree in virology. Instructor and associate researcher, Pedro Kourí Tropical Medicine Institute, Havana, Cuba.
Licel de los Angeles Rodríguez Lay, physician with a doctorate in medical sciences. Full professor and senior researcher, Pedro Kourí Tropical Medicine Institute, Havana, Cuba.
Susel Sariego Frómeta, virologist with a master degree. Research candidate and instructor, Pedro Kourí Tropical Medicine Institute, Havana, Cuba.
Denis Verdasquera Corcho, physician with a master’s degree in infectious diseases. Associate professor and associate researcher, Pedro Kourí Tropical Medicine Institute, Havana, Cuba.
Marguerite Vincent, biologist. Pedro Kourí Tropical Medicine Institute, Havana, Cuba.
Aidonis Gutiérrez Moreno, chemistry technician. Pedro Kourí Tropical Medicine Institute, Havana, Cuba.
Meilin Sánchez Wong, chemistry technician. Pedro Kourí Tropical Medicine Institute, Havana, Cuba.
Submitted: July 1, 2010 Approved: March 1, 2011








