INTRODUCTION
Sepsis is the most common direct cause of death worldwide, and thus is considered a significant health problem, equally affecting children and adults.[1,2] It is a clinical syndrome caused by physiological changes indicating systemic inflammation from suspected or proven infection. Septic shock is the progression of these changes to the point where they compromise delivery of oxygen and metabolic substrates to the tissues.[3]
Several research teams are applying genetics and gene biology to reproductive cells as a way of developing new therapeutic strategies, seeking to identify sepsis markers and subclasses of septic shock. Diverse polymorphic genes extensively involved in inflammation, immunity and coagulation have been linked with susceptibility to sepsis.[4–7]
Pediatric septic shock is currently defined as sepsis accompanied by cardiovascular dysfunction, with or without hypotension,[8] recognizable before it occurs by a clinical triad of hypo- or hyperthermia, altered mental state, and peripheral vasodilation (warm shock) or cold extremities (cold shock).[9]
Globally, some 29,000 children aged < 5 years die of sepsis daily.[10] In the USA, severe sepsis and septic shock are responsible for about 4500 deaths each year and almost US$2 billion annually in medical expenditures.[11] A study published in 2001 put US case fatality at 34.1%.[12]
In 2002, the American College of Critical Care Medicine (ACCM) published Clinical Guidelines for Hemodynamic Support of Neonates and Children in Septic Shock, aiming to reduce case fatality.[9] The Guidelines prescribe measures for early recognition of septic shock and goal-directed therapies to optimize patient management during the first hour, including aggressive fluid resuscitation, insulin therapy to maintain normal glucose levels, steroids for patients at risk of absolute adrenal insufficiency, lung-protective ventilation in cases of acute respiratory distress syndrome, and dialysis if needed. Centers using the Guidelines report case fatality rates of 8–13% (1–3% in previously healthy children and 7–10% in chronically ill children).[13,14]
A 2001–2006 report found 14.6% case fatality in 178 sepsis patients in the pediatric intensive care unit (PICU) of the Luis Díaz Soto Military Hospital in Havana, and 53.8% case fatality for septic shock;[15] while in the PICU of José Luis Miranda University Pediatric Hospital (HPU, the Spanish acronym) in Santa Clara, Villa Clara Province, septic shock case fatality was 26.3% (unpublished hospital data).
In 2002, case fatality from septic shock in HPU PICU was 35.2%. HPU began implementing the ACCM Guidelines in the last quarter of that year, observing a reduction in case fatality from 34.6% to 19% between 2003 and 2007 (unpublished data). The purpose of this 2008–2010 study is to describe implementation of the updated ACCM Guidelines[16] and discuss their possible impact on case fatality.
METHODS
Type of study and patients A descriptive study was conducted from January 2008 through December 2010. Subjects were 280 male and female patients, newborn through 18 years, admitted to HPU’s PICU with a diagnosis of septic shock. HPU, the referral hospital for Cuba’s central provinces, has 321 beds, 10 in PICU, and provides care in 18 clinical and 8 surgical pediatric specialties.
Diagnostic and therapeutic criteria
Diagnostic The study used criteria described in the ACCM Guidelines of June 2002,[9] updated in 2007.[16] It also took into account the findings of the 2001 International Sepsis Consensus Conference,[17] its diagnostic criteria adapted for sepsis in children; and those of the 2005 International Pediatric Sepsis Consensus Conference, which defined sepsis and organ dysfunction—[18]definitions that help differentiate the clinical phases of sepsis, fundamentally between severe sepsis and septic shock.
Therapy Fluid replacement therapy was initially administered through one or two peripheral veins, or via intraosseous infusion when peripheral access was unfeasible, and subsequently, through a central venous catheter through the jugular, subclavian, or femoral vein, when necessary. A 0.9% saline solution was used as crystalloid therapy in all patients in the first two boluses, and human albumin in 5% saline solution or gelatin as colloid therapy in the third bolus in patients showing little improvement with the first two.
Vasoactive drugs used as inotropic agents included dobutamine (DBT) as a dopamine substitute, since it can be safely administered through a peripheral vein; catecholamines (epinephrine, EPI; norepinephrine, norEPI); and vasodilators (nitroglycerin, NTG) and related drugs.
Variables The dependent variable was case fatality, defined as percentage of deaths at 28 days. Independent variables are described in Table 1. On arrival in PICU, all patients were assessed to determine whether they were in septic shock, and classified clinically by hemodynamic state (Table 1). Criteria for myocardial dysfunction or myocardial damage secondary to sepsis were corroborated by echo- and electrocardiography.
Data collection and analysis A form was designed to compile patient data during admission, to avoid having to refer to clinical history after patient discharge from PICU. SPSS 19.0 for Windows was used for data processing. Results were summarized in statistical tables with absolute and relative frequencies. Risk was estimated by calculating odds ratios (OR) with their 95% confidence intervals, and statistical significance assessed with Fisher’s exact test, with a threshold of p <0.05.
Ethics Written informed consent was obtained from the children’s guardians, with assurance of patient anonymity. The study was approved by the HPU medical ethics committee.
RESULTS
In 2008–2010, patients with a diagnosis of septic shock accounted for 14.8% of PICU admissions (280/1894). A total of 31 patients died, for an overall 28-day case fatality rate of 11.1%. Infants aged 1 month to 1 year were the largest age group diagnosed (55%) but had the lowest case fatality, 7.1%, and OR for death versus all other ages was 0.41 (95% CI: 0.17–0.94, p = 0.02). There were more boys than girls diagnosed, 61.1% vs. 38.9%, but girls had higher case fatality, 13.8% vs. 9.4% (p = 0.342) (Table 2).
Children with comorbidities were 16.1% of patients (45/280), but accounted for 45.2% of deaths (14/31), with case fatality of 31.1%, vs. 7.2% (17/235) in previously healthy children. OR for death in children with comorbidities versus healthy children was 5.79 (95% CI: 2.42–13.88, p <0.001). A breakdown of patients by age (<2 years or ≥2 years) showed that 33.3% (15/45) were in the group aged <2 years, with 4 deaths, for 26.7% case fatality (OR 3.21, 95% CI: 0.80–11.9, p = 0.070); and 66.7% (30/45) in the group aged ≥2 years, with 10 deaths, for 33.3% case fatality (OR 5.45, 95% CI: 2.07–14.3, p<0.001) (Table 2).
Most cases of sepsis (78.6%) originated in the community, but case fatality was higher in cases of nosocomial origin (23.3% vs. 7.7%, p = 0.001) (Table 3).
Cold shock with low cardiac output (CO)/high systemic vascular resistance (SVR) was the most frequent hemodynamic state (68.9%), followed by warm shock with high CO/low SVR (18.6%). Cold shock with low CO/low SVR was less common (12.5%), but had a higher case fatality, 34.3% (OR 6.21, 95% CI: 2.47–15.57, p <0.001) (Table 3).
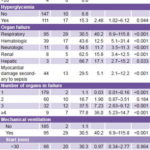
Glucose levels were tested in only 258 patients, with hyperglycemia found in 111, or 43%; there were 17 deaths in the hyperglycemic group, for a case fatality of 15.3% vs. 6.8% in patients with normal glucose levels (OR 2.48, 95% CI 1.02–6.12, p = 0.04). When hyperglycemia was in the 10–15.9 mmol/L range, case fatality was 25.6% (OR 3.55, 95% CI: 1.08–12.04, p = 0.03); at higher glucose levels than this, very small numbers (n of 2 and 4) making significance testing moot (Table 4).
Apart from cardiovascular dysfunction—present in all patients by definition—respiratory (34%) and hematologic (13.9%) failure predominated. Sepsis-associated myocardial damage was found in 15.7% of patients (OR 5.1, 95% CI: 2.1–12.2, p < 0.001). Case fatality increased with number of dysfunctional organs, up to 77.8% (7/9 patients) when 4 or more were involved (OR 36.02, 95% CI: 5.23–14.74, p <0.001) (Table 4).
Mechanical ventilation was used with 33.9% (95/280) of shock patients, with 29 deaths, for a 30.5% case fatality (OR 40.295% CI 6.88–115.8, p < 0.001). In 69.5% (66/95) of those ventilated, the procedure was initiated within 30 minutes of admission, and in 81.1% within the first hour. Two patients were intubated but died before they could be connected to a ventilator. Some 33.7% of patients (32/95) were ventilated for 3–5 days, and 29.5% (28/95) for >10 days (Table 4).
In the first hour of treatment, 90% of patients (252/280) received fluid therapy, with volumes of 40–100 mL/kg. During this first hour, no patient received >100 mL/kg and 28 received < 40 mL/kg. In the first 3 hours, 96.4% of patients (270/280) received 40–100 mL/kg, 1.1% (3/280) received >100 mL/kg, and 2.5% (7/280) received < 40 mL/kg. By six hours, 96.1% of patients (269/280) had received 40–100 mL/kg and 3.6% (10/280) >100 mL/kg; a single patient (0.4%) received < 40 mL/kg. There were no complications of respiratory distress syndrome or cerebral edema secondary to fluid administration.
Table 1: Independent variables

BP: blood pressure / CO: cardiac output / DBT: Dobutamine / EPI: Epinephrine / norEPI: norepinephrine / NTG: nitroglycerin / PaCO2: partial pressure of arterial carbon dioxide / PO2/FiO2: oxygen pressure/fraction of inspired oxygen / SVR: systemic vascular resistance
Table 2: Population characteristics and comorbidities
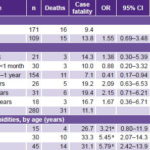
avs. all other children / bvs. healthy children, all ages
Table 3: 28-day case fatality by sepsis origin and shock hemodynamic type
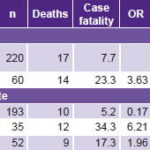
CO: cardiac output / SVR: systemic vascular resistance
In combination drug therapy for cold shock (low CO/high SVR), the most frequently used drugs were DBT+EPI, the latter at a low dose of 0.05–0.3 µg/kg/min. In this same hemodynamic state, but with catecholamine-resistant, vasodilator-responsive shock, combinations of EPI+NTG and EPI+NTG+DBT were used, this last in patients with associated severe myocardial damage. The most frequently used dose of EPI was 0.1–0.3 µg/kg/min and of NTG, 5–7.5 µg/kg/min.
In cold shock (low CO/low SVR), the most frequent combination was DBT+norEPI, the latter at doses of 0.1–1.0 µg/kg/min; in only one patient was a dose higher than 1 µg/kg/min used. The DBT+EPI combination was used in only 5 patients, the dose of the latter >0.4 µg/kg/min. In warm shock, (high CO/low SVR), the most frequent combination was DBT+norEPI, dose of the latter 0.1–0.6 µg/kg/min; in only one patient the dose used was 1 µg/kg/min (Table 5).
The most common therapeutic response was fluid refractory, DBT responsive, 39.3% (110/280), DBT being the vasoactive drug used as the main inotropic agent at 10 µg/kg/minute; the next most common response was fluid-refractory, DBT resistant, responsive to catecholamines in 26.4% (74/280). Although refractory shock constituted only 6.1% of cases (17/280), it accounted for 54.8% of deaths (17/31), with 100% case fatality (Table 5).
Table 4: Case fatality by glucose level, organ dysfunction and mechanical ventilation use
A total of 14 patients recovered from septic shock but died of other causes before 28 days. Of these, 11 suffered from chronic conditions or comorbidities (OR 6.5, 95% CI 2.69–15.7, p < 0.001). Of the same 14, 12 developed organ dysfunction secondary to sepsis; 10 had comorbidities, including hematologic cancer (5), infantile cerebral palsy (3), and high-risk congenital malformations in newborns (2); 2 without comorbidities had central nervous system infections. There were two deaths from accidents during emergency medical care (central venous catheterization and tracheostomy decannulation).
DISCUSSION
The 28-day case fatality rates we observed are within the range found in other hospitals that have similarly applied ACCM Guidelines.[14] The Han study reports an association between early use of ACCM Pediatric Advanced Life Support Guidelines and improved outcomes in newborns and children (case fatality 38% without vs. 8% with).[19] Menif reports a similar experience with optimal management of critical shock patients within the first hour, noting that each hour that passes without restoring normal (for age) blood pressure and capillary refill to less than three seconds is associated with a twofold increase in case fatality.[20] The Sophia Children’s Hospital in Rotterdam recently reported a substantial reduction in case fatailty from purpura and severe sepsis after implementation of 2002 guideline-based therapy in the referral center, transport system, and tertiary care settings.[21] This center also used high flux continuous renal replacement therapy and fresh frozen plasma infusion aimed at achieving normal prothrombin time (international normalized ratio).
Table 5: Case fatality by therapeutic response and drugs utilized

CO: cardiac output / DBT: dobutamine / EPI: epinephrine / norEPI: norepinephrine / NTG: nitroglycerin / SVR: systemic vascular resistance
The statistically significant predominance of infants versus other age groups is common, and can be explained by their immunologic immaturity.[22] The male:female ratio observed (1.6:1) was slightly higher than reported in a Brazilian hospital (1.3:1, with 56.1% of sepsis in males) and lower than reported in Panama City (male:female ratio of 2:1, with 67% of sepsis in males).[23]
Our observed case fatality for patients with chronic conditions was higher than reported for some international institutions that followed the 2007 Guidelines (7%–10%).[16,24] In the most important epidemiological study of pediatric sepsis, Watson reviewed the discharge databases of 942 hospitals in the USA and found higher case fatality in patients with severe sepsis who had comorbidities, from 14.2% in children aged 10–19 years to 58.7% in aged 1–9 years, but provided no analysis of Guideline compliance. The higher rates in the latter could be explained by greater proportions of children with comorbidities from conditions such as cancer, congenital birth defects, cerebral palsy and end-stage renal disease.[25]
In our study, community-acquired infection predominated, which is not surprising, given the higher proportion of previously healthy people in the general population; however, a higher risk of death was associated with nosocomial sepsis, owing to the aggressiveness and more powerful resistance mechanisms of hospital microorganisms. In his retrospective study (1981–1992) of children with sepsis in a Panama City hospital, Sáez-Llorens found that 60% of infections were community acquired (case fatality of 36.9%) and 49% nosocomial (case fatality of 42.5%).[26] On the other hand, a multicenter study of adults in Germany (2075 ICUs in 1380 hospitals) found that only 39.1% of sepsis was community acquired, while 46.7% was nosocomial (32.9% in ICUs, 13.8% in other services, 14.2% in undetermined locations).[27]
As Ceneviva points out, once treatment for shock has begun, septic hemodynamic states can progress and change, especially in the first 48 hours; he reports on 50 children with dopamine-resistant (≥60 mL/kg in the first hour) fluid-refractory shock, most of whom (58%) had cold shock with low CO/high SVR; 22% had low CO/low SVR; and only 18% had warm shock with high CO/low SVR. These figures are similar to ours, in which cold shock with low CO/high SVR also predominated.[28]
Furnary and Braithwaite note that hyperglycemia is frequently observed in gravely ill patients; it has many causes, but is most often associated with metabolic stress.[29]
Sepsis is characterized by marked insulin resistance, directly proportional to severity of stress response. In 2004, the Surviving Sepsis Campaign recommended glucose monitoring for all patients with sepsis.[30] This recommendation was retained in the 2008 and 2013 updates[31,32] and has led to worldwide adoption of strict glucose monitoring in ICUs.
Brierley notes that children in septic shock with hyperglycemia of >140 mg/dL and an elevated anion gap exhibit resolution of this gap when insulin is added to their regimen, observing a reduction in catabolism.[16] In a similar study of pediatric patients in septic shock, Branco shows outcomes that coincide with ours, reporting an increase in the risk of death with glycemia >9.9 mmol/L.[33] In another prospective observational study of children with meningococcal septic shock, Verhoeven found hyperglycemia of >8.3 mmol/L in 33% of patients.[34]
There are few studies of organ dysfunction in children; in a retrospective study (1990–1995), Mora found that the syndrome is common and case fatality very high, with a risk of death of 25%–52% in children with severe sepsis and septic shock. This figure is compatible with our findings.[35] In a Lima, Peru PICU, Tantalean found that case fatality increased in direct proportion to number of organs in failure, from 29.4% for 2 organs to 100% for ≥5.[36] In a prospective study in a Malaysian PICU, Goh found a case fatality rate of 57% for patients admitted with any organ failure, compared with 0.5% in patients without organ dysfunction. Case fatality increased directly with the number of organs in failure (from 44% for 2 organs to 92% for ≥4).[37]
Brierley cites several reasons for starting mechanical ventilation early in patients with septic shock, since up to 40% of cardiac output may be required to support respiration, and sedation and analgesia combined with ventilation facilitate temperature control and reduce oxygen consumption.[16] Therefore, any patient not reaching rapid stabilization with peripherally administered fluids and inotropic agents should be put on mechanical ventilation. Less commonly, mechanical ventilation is required for patients with respiratory failure, deteriorating mental state, or in extremely critical condition. These criteria are consistent with those utilized in this study; patients requiring this treatment were at high risk of death, as the outcomes indicate.
It was not until 1998 that researchers reported outcomes of using aggressive volume resuscitation in children with septic shock (60 mL/kg of fluid in the first hour), with the therapeutic goal of obtaining a cardiac index of 3.3–6.0 L/min/m2 and normal pressure in the pulmonary capillary bed.[38] Since 2002, several randomized studies of patients with dengue shock syndrome,[39] WHO grade III (narrow pulse pressure/tachycardia) and some with grade IV (hypotension), who received aggressive fluid resuscitation have observed a survival rate of nearly 100%, regardless of fluid used.
In our study, similar fluid volumes were aggressively administered without complications, a result also observed by Brierley, who reports that none of the children who were administered large fluid volumes to stabilize them developed respiratory distress syndrome or cerebral edema.[16]
These outcomes differ from those of Maitland, who reports that after boluses of fluid were administered to African children with severe infections, there was a 2–3% increase in pulmonary edema and intracranial pressure, with a slight but statistically insignificant increase in relative risk. It should be noted that >55% of these patients had malaria, a disease commonly associated with neurologic and pulmonary complications.[40]
Chopra’s randomized study compared the efficacy of 3% saline solution and 0.9% solution in initial fluid resuscitation therapy administered to 60 children aged 2–12 years with septic shock. The amount of fluid required for resuscitation with 3% saline solution was roughly half that required with 0.9% solution, but use of vasopressors, time taken to resolve shock, length of stay, and PICU case fatality were similar in both groups, leading to the conclusion that the two fluids are equally effective in restoring hemodynamic stability and maintaining average length of stay in PICU, with similar case fatality rates.[41] In our study, the only crystalloid fluid used was 0.9% saline solution, with satisfactory outcomes.
Among vasoactive drugs, dopamine/DBT (5–9 μg/kg/min) or EPI (0.05–0.3 μg/kg/min) can be used as first-line inotropic support, DBT especially in cases of low CO with adequate or increased SVR.[22] Kissoon states that inotropic agents should be administered via peripheral venous or intraosseous access when central venous access is unavailable, because delayed administration of inotropic agents can substantially increase risk of death.[42] These criteria were employed in our study, using DBT as the main inotropic agent because it can be administered through a peripheral vein, given difficulties with venous access in children, especially newborns and infants.
In managing children with normal blood pressure in fluid-refractory dopamine/DBT-resistant cold shock and low CO/high SVR, use of an inotropic agent like EPI that lowers SVR is recommended, along with a fast-acting vasodilator such as sodium nitroprusside or NTG, to restore microcirculation and reduce afterload; this results in improved ventricular ejection and overall cardiac output, particularly when ventricular function is compromised,[43–46] a criterion also used in this study.
Sakr notes that even though dopamine is still considered the first-line vasopressor for fluid-refractory hypotensive shock and low SVR, there is evidence that patients treated with this drug have poorer outcomes.[47] Liet has also noted that dopamine resistance is common in infancy; sympathetic innervation in immature animals and young humans (newborns, preterm infants, and infants < 6 months) may not have fully developed, resulting in reduced norEPI release from their reserves.[48]
Dopamine-resistant shock commonly responds to norEPI or high doses of EPI.[29] Dopamine was not used as a vasopressor in this study; this effect was obtained with early use of norEPI, although at low doses, for fluid-refractory hypotensive hyperdynamic shock, as recommended by Morimatsu.[49] Hall recommends use of DBT with norEPI, noting that it is a potent inotropic agent with intrinsic vasodilating action that can be useful in counteracting norEPI’s excessive vasoconstriction.[50]
Improved capillary and intestinal blood flow have been observed in animal and human studies with norEPI plus DBT in comparison with high doses of dopamine or EPI.[51] The drug combinations used in our study follow the criteria indicated by these authors.[49–51]
Our results suggest that implementation of ACCM Guidelines to achieve early diagnosis and swift initiation of goal-directed therapy has contributed to lowering case fatality from septic shock in HPU’s PICU, which fell from 34.6% in 2003 to 19% in 2007 (administrative data), and to 11.1% in the 3-year period of this study. Examples of actions taken within these Guidelines are optimal management in the first hour, early and aggressive fluid resuscitation, support with vasoactive drugs singly or in combination, early use of mechanical ventilation as necessary, and insulin therapy to maintain normal blood glucose levels.[9,16,20]
The main limitations of this study are its ecological design and short duration, which did not permit research on case fatality over a longer period or confirmation of how implementing ACCM Guidelines affected the downward trend. However, the experience gained has been critical for saving the lives of young patients. Another equally important limitation is the lack of patient assessment using pediatric scores that predict death and organ dysfunction (PRISM, PELOD).[51,52]
CONCLUSIONS
The ACCM Guidelines are a valuable instrument for timely, appropriate care of pediatric patients in septic shock: early aggressive fluid therapy, supported by vasoactive drugs singly and in combination, can have a positive impact on clinical course. Multicenter studies with larger numbers of patients are needed to corroborate these observations and further improve management of pediatric shock.








