ABSTRACT
INTRODUCTION By the end of 2017, there were more than 28,000 individuals living with HIV in Cuba, over 80% receiving antiretroviral therapy, which dramatically reduces viral replication, improves immune status and decreases risk of transmission. These results could be jeopardized by emergence of HIV-1 drug resistance. In 2009, a test for HIV-1 genotypic resistance was introduced in routine clinical practice in Cuba.
OBJECTIVE Investigate antiretroviral resistance and its relation to subtype distribution in HIV-1 treatment-naïve and previously treated patients in Cuba.
METHODS Resistance and HIV-1 subtype distribution were determined in 342 antiretroviral treatment-naïve patients and 584 previously treated for HIV-1 whose blood specimens were sent to the Pedro Kourí Tropical Medicine Institute during 2009–2014. Transmitted drug resistance was determined using the Calibrated Population Resistance Tool v.6. Drug resistance analysis was conducted using the algorithm Rega v9.1.0.
RESULTS Prevalence of transmitted drug resistance was 11.4%, and 41% of mutated viruses exhibited dual-class resistance to nucleoside reverse transcriptase inhibitor and non-nucleoside reverse transcriptase inhibitor. Overall, 84.9% of patients had ≥1 resistance mutation, 80% had ≥1 nucleoside reverse transcriptase inhibitor mutation, 71.4% had ≥1 non-nucleoside reverse transcriptase inhibitor mutation and 31.7% had ≥1 protease inhibitor mutation. K65R and K101E mutations were significantly more frequent in subtype C, L210W in CRF19_cpx, and M47V/I in CRF BGs (20, 23, 24). Full class resistance to nucleoside reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors, protease inhibitors and multidrug resistance were detected in 21.2%, 32.4%, 8% and 4.1% of patients, respectively. Average percentage resistance to nucleoside reverse transcriptase inhibitor, protease inhibitor, full class resistance to nucleoside reverse transcriptase inhibitor, protease inhibitor and multidrug resistance increased in patients failing two or more regimens. Nevertheless, after 2011, a declining trend was observed in the frequency of multidrug resistance and full class resistance to nucleoside reverse transcriptase inhibitors and protease inhibitors.
CONCLUSIONS Detected levels of transmitted drug resistance highlight the need for a national surveillance study in treatment-naïve patients. Resistance prevalence is high in previously treated patients but appears to be decreasing over time. The frequency of resistance mutations in recombinant forms of HIV in Cuba needs further study.
KEYWORDS Antiretroviral therapy, highly active antiretroviral therapy, HIV, anti-HIV agents, drug resistance, multiple drug resistance, Cuba
INTRODUCTION
There is a global and regional commitment to reach the Joint United Nations Programme on HIV/AIDS’ 90–90–90 target in 2020, and to end AIDS by 2030.[1] The 90–90–90 target is that 90% of all people living with HIV will have been diagnosed, 90% of all people with known HIV infection will be receiving antiretroviral therapy (ART), and 90% of all people receiving ART will have a suppressed viral load. Latin America and the Caribbean region face major challenges in meeting this target. PAHO has reported substantial progress (continuing decline in AIDS-related deaths and mother-to-child HIV transmission, increasing numbers of people who know their HIV status and receive treatment), but the annual number of new infections in the Caribbean has remained static since 2012 and HIV incidence remains high in key populations, mainly men who have sex with men (MSM) and transgender women.[1,2]
IMPORTANCE This study shows high levels of resistance to antiretroviral drugs used in Cuba up to 2014, indicating an urgent need for changes in first-line therapy. It also reinforces the necessity of resistance testing for all patients failing antiretroviral therapy.
In 2001, Cuba’s Ministry of Public Health (MINSAP) decided to produce generic drugs for treatment of HIV. Efforts to provide access to ART have accelerated since then and have resulted in decreased AIDS mortality and incidence of opportunistic infections.[3,4] By the end of 2017, >28,000 individuals were living with HIV in Cuba, >90% of infected individuals were aware of their HIV status and approximately 80% were on ART. However only half of patients in treatment were virally suppressed (information from MINSAP’s National HIV Registry, 2016), a major gap for Cuba in meeting the third 90–90–90 target.
ART dramatically reduces viral replication, improves immune status and decreases risk of HIV transmission, but these outcomes could be jeopardized by HIV-1 resistance. A 2004 study that explored ART resistance in Cuba found low levels of resistance.[4] In 2009 the Pedro Kourí Tropical Medicine Institute (IPK) introduced an in-house HIV-1 genotyping system for routine assessment of drug resistance in Cuban patients.[5]
The aim of this research was to investigate the frequency and profile of antiviral drug resistance in HIV-1 treatment-naïve and previously treated patients and estimate the prevalence of specific resistance mutations among HIV-1 variants circulating in Cuba.
METHODS
Population IPK is the reference center for HIV care and therapy in Cuba, thus samples from all over Cuba are sent to IPK for genotypic drug resistance testing. A total of 926 viral sequences were collected of all HIV-1 genotypic drug resistance testing carried out at IPK’s laboratory as part of routine clinical care from April 2009 to December 2014. One sample per patient was analyzed from 584 previously treated patients and 342 treatment-naïve individuals. Only epidemiologic, demographic, clinical, virological and immunological data were collected; no patient identifying information was retained.
Viral load and CD4 count Plasma HIV-1 viral loads were determined using the Nuclisens Easy Q HIV-1 kit v2.0 (Biomérieux, France) or COBAS Ampliprep/COBAS Taqman HIV-1 test v2.0 for use with the High Pure System (Roche, Germany). CD4 cell counts were determined using a Becton Dickinson counter (Bio-Sciences, USA).
Genotypic drug resistance testing For HIV-1 genotyping, 1 mL plasma was ultracentrifuged and the suspended pellet extracted using QIAamp Viral RNA Kit (QIAGEN, Germany) manually, or automatically on QIAcube (QIAGEN, Germany), per manufacturer’s protocol. HIV-1 RNA reverse transcription, amplification and population-based bidirectional Sanger sequencing of pol fragments were carried out as described elsewhere.[5] Sequences obtained covering a fragment of 1302 bp that overlaps with codons 1–99 of protease and 1–335 of reverse transcriptase were edited and assembled using Sequencher, v4.1 (Gene Codes Corporation, USA).
Data analysis HIV subtype was determined using Rega subtyping tool version 3 and confirmed by manual phylogenetic analysis, using MEGA v6 (Kimura’s 2-parameter correction, bootstrap 1000).[6]
Therapeutic failure was defined as ART failure to reduce and maintain viral load at <200 copies/mL. Information about treatment compliance was unavailable.
Prevalence of genotypic drug resistance mutations in treatment-naïve patients was analyzed using the Calibrated Population Resistance Tool v6 and based on WHO’s 2009 surveillance of drug-resistant mutations.[7]
Drug resistance interpretation in previously treated patients was conducted using the resistance interpretation algorithm Rega v9.1.0. Resistance to drug classes was calculated by averaging the percentage of resistance (R) and intermediate resistance (I) for each drug class. Full-class resistance (FCR) was defined as lack of full susceptibility to any antiviral drug in a given drug class.[8] Multidrug resistance (MDR) was scored if the virus strain was susceptible to no more than one drug belonging to the three commonly available drug classes in Cuba.[8] For statistical analysis, chi square with Yates correction, Fisher exact test and odds ratios (OR) were calculated using Epidat v3.0.10.[9]
Ethics The study was approved by the IPK Ethics Committee and complies with the Declaration of Helsinki.[10] At time of collection, all subjects included in the study gave written informed consent for their specimens to be used for research purposes.
RESULTS
Study population Participants were predominantly male (83.3%), MSM (76.8%) and resided in Havana (66.1%). Median age was 32.4 years (interquartile range, IQR: 24.6–41.3) and 40.5 years (IQR: 33.6–46.6) for treatment-naïve and previously treated patients, respectively. Median CD4 cell count in treatment-naïve patients was higher than in previously treated patients (349 cells/mm3 vs 208 cells/mm3), but viral loads were similar in both groups (18,966 copies/mL and 21,264 copies/mL, respectively) (Table 1).
Mean time since ART initiation was 3 years (IQR: 1.1–5.6). All patients had received nucleoside reverse transcriptase inhibitors (NRTI); 90.1% had received ≥1 non-nucleoside reverse transcriptase inhibitors (NNRTI) and 62.7% had received ≥1 protease inhibitor (PI). Only 12.8% of patients had received mono- or dual therapy regimens. At the time of drug resistance testing, the most commonly prescribed drugs were lamivudine (3TC), 92.5%; zidovudine (AZT), 44.7%; and nevirapine (NVP), 44.2%.
Subtype distribution In the study period, 30.9% of HIV-1 strains were subtype B, 22% were BG recombinants (CRF20_BG, CRF23_BG and CRF24_BG), 18.3% CRF19_cpx, 9.8% CRF18_cpx, 6.5% URF, 5.5% subtype C, 2.3% subtype G and 4.7% were other subtypes with frequencies <1% (subtypes A, F, J, H; CRF02_AG, CRF06_cpx, CRF14_BG and CRF31_BC). There were no significant differences between HIV-1 subtypes identified in samples from treatment-naïve and those from treatment–experienced patients (Table 1).
Drug resistance in treatment-naïve patients Overall, 11.4% (39/342) of treatment-naïve HIV-1 patients showed evidence of transmitted drug resistance (TDR). The frequency of single TDR against NRTI was 20.5%, against NNRTI 12.8% and against PI 17.9%, for a total of 51.2% single drug class resistance. High prevalence of dual-class resistance was observed (43.6%), mainly to NRTI+NNRTI (41%). Triple drug class resistance was observed in 2 patients (5.1%) (Table 2).
The most common mutations related to NRTI resistance were M184V/I (46.2%), T215Y/I/S/D (25.6%) and K219Q/E/N/R (20.5%); for NNRTI were K103N (23.1%) and Y181 C/I (28.2%) while for PI was M46I/L (15.4%) (Table 2).
No significant differences were observed in overall TDR mutation frequency between chronically infected patients (48.7%) and recently diagnosed individuals (51.3%). However, TDR to NRTI was higher in chronically infected individuals. In contrast, TDR against NNRTI and PI was higher in recently diagnosed individuals. Mutation M184V/I was more frequently detected among chronically infected individuals (p = 0.0390, OR 4.0, 95% CI 1.0–15.2) (Table 2).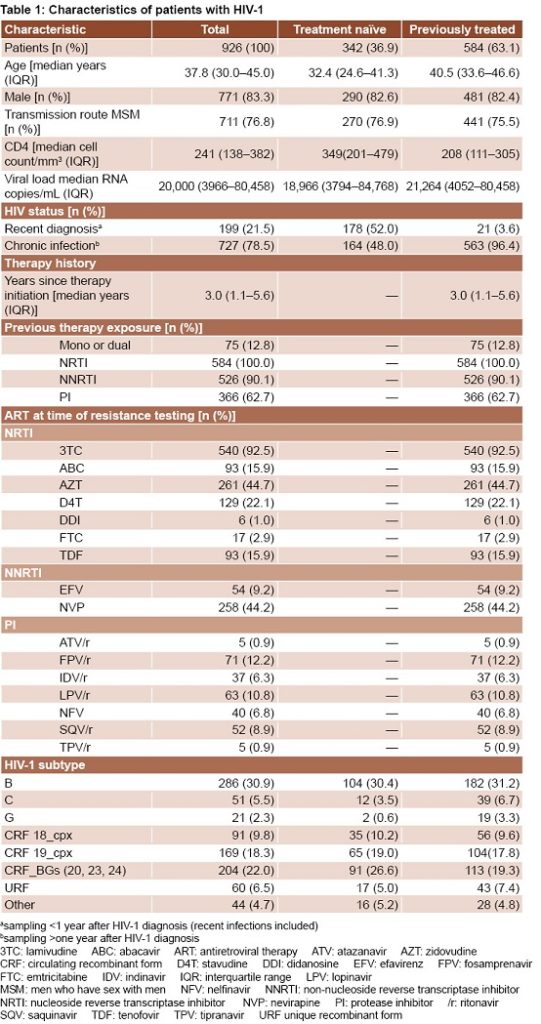
Drug resistance mutations in previously treated patients Overall, 84.9% of patients had ≥1 resistance mutation, 80% had ≥1 NRTI mutation, 71.4% had ≥1 NNRTI mutation and 31.7% had ≥1 PI mutation. The most frequent NRTI mutations were M184V/I (75.9%), T215Y/F (37.3%), and M41L (25.7%). The most frequent NNRTI mutations were K103N/S (28.6%), Y181C/I/V (26.4%) and G190S/A (21.7%). The most common PI mutations were L90M (16.3%), M46I/L (15.9%) and V82A/T/F/S (10.3%) (Table 3).
Frequency of drug resistance mutations to any drug class was significantly higher in patients who had undergone ≥3 therapy regimens (p = 0.0149, OR 2.1, 95% CI 1.1–3.8) compared to those with fewer regimens. Mutations associated with NRTIs, NNRTIs and PIs were observed in 74.4%, 69.9% and 9.7% of first-line failures, respectively. In patients failing second-line therapy, the respective frequencies were 79.2%, 72.2% and 31.9%. In patients exposed to ≥3 ART regimens, these values increased to 84.1%, 72% and 46.2%, respectively. For each specific NRTI and PI mutation, significant differences were observed between patients exposed to 1 or 2 regimens compared to those exposed to ≥3 regimens (p <0.05). The number of patients harboring viruses with NNRTI mutations did not significantly increase in those exposed to ≥2 regimens, but the frequency of K103N/S and V108I was higher (p = 0.0402 and p = 0.0049, respectively) in patients exposed to ≥3 than in patients failing the first regimen. Dual-class resistance mutations to NRTI+NNRTI were more frequently observed in patients exposed to 2 therapies (p = 0.0017, OR 2.0, 95% CI 1.3–3.2) compared with first therapy failures. The same was observed for dual-class resistance to NRTI+PI (p <0.001, OR 14.2, 95% CI 6.6–30.5) and for triple class resistance (p = 0.0067, OR 2.0, 95% CI 1.2–3.4) (Table 3).
Prevalence of resistance mutations among different subtypes in patients on active ART As shown in Table 4, NRTI re sistance mutation K65R was significantly more frequent among subtype C isolates from patients treated with 3TC whereas L210W was present in higher proportions among CRF19_cpx isolates from individuals failing AZT or stavudine (D4T) regimens. NNRTI resistance mutation K101E was more frequent in subtype C isolates from patients failing NVP therapy. PI mutation M47V/I was more frequent among recombinant forms CRF_BGs (20, 23, 24) isolates from patients failing LPV/r therapy.
sistance mutation K65R was significantly more frequent among subtype C isolates from patients treated with 3TC whereas L210W was present in higher proportions among CRF19_cpx isolates from individuals failing AZT or stavudine (D4T) regimens. NNRTI resistance mutation K101E was more frequent in subtype C isolates from patients failing NVP therapy. PI mutation M47V/I was more frequent among recombinant forms CRF_BGs (20, 23, 24) isolates from patients failing LPV/r therapy.
Drug resistance prevalence and trends in previously treated patients The highest drug resistance levels against NRTI were detected for 3TC/FTC (76.9%) and ABC (50.2%); against NNRTIs were for NVP (71.2%) and EFV (70.9%); against PI were NFV (31.8%) and SQV/r (26.4%) (Figure 1a).
The average proportions of patients harboring NRTI, NNRTI and PI resistance were 52.7%, 54.7% and 21.4%, respectively. This average significantly increased in patients failing ≥2 regimens for NRTI (p <0.0001) and PI resistance (p <0.0001). FCR to NRTI, NNRTI and PI was observed in 21.2%, 32.4% and 8%, respectively. FCR to NRTI and PI also significantly increased after two regimens failures (p = 0.0001 and p <0.0001, respectively). MDR was present in 4.1% of studied patients and significantly increased aft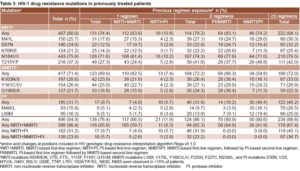 er two regimens failures (p = 0.0017) (Figure 1b).
er two regimens failures (p = 0.0017) (Figure 1b).
From 2009 to 2014, a significant declining trend in MDR prevalence was noticed. In 2009 12.6% of patients harbored an MDR virus, whereas in 2011 prevalence fell to 2.3% (OR = 0.80, 95% CI 0.69–0.93; p = 0.003) (Figure 2a). Furthermore, a significant decline was observed for FCR NRTI and FCR PI. Statistical analysis demonstrated that FCR NRTI is significantly decreasing over time, from 37.6% in 2009 to 9.5% in 2014 (OR = 0.74, 95% CI 0.70–0.82; p <0.001). For FCR PI, a significant decrease was also observed between 2009 and 2012, from 24.7% to 0.8% (OR = 0.80, 95% CI 0.71–0.89; p <0.001). When this analysis was performed to include any drug in each drug class, PI resistance showed a similar declining trend (p <0.001) (Figure 2b).
DISCUSSION
These results describe circulating subtypes and prevalence of drug resistance for HIV-1 infections in Cuba during 2009–2014. The finding that HIV-1 non-B subtypes were more frequent is consistent with previous studies[6,11–14] and in contrast with the high proportion of subtype B reported in the Caribbean.[15,16] The broad genetic diversity of HIV-1 in Cuba is thought to be due to its originating from contacts in Central Africa.[14,17,18]
Cuba has made great strides in decreasing HIV-related morbidity and mortality by providing universal free access to ART.[11] Because of economic constraints, the most common drug combinations for first-line ART are restricted to nationally manufactured generic drugs.[12] Drug resistance testing was not available until May 2009, so a substantial number of patients may have been treated with failed virological regimens.[6]
The hi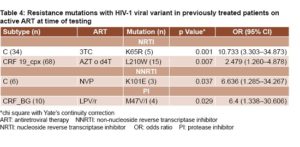 gh overall TDR prevalence detected confirms previous reports in Cuba,[11,19] and is higher than reported in other Caribbean countries, Mexico and Central America.[20–26] Particularly alarming is the frequent detection of dual-class resistance to NRTI+NNRTI, since these classes of drugs constitute the backbone of first-line therapy in Cuba.
gh overall TDR prevalence detected confirms previous reports in Cuba,[11,19] and is higher than reported in other Caribbean countries, Mexico and Central America.[20–26] Particularly alarming is the frequent detection of dual-class resistance to NRTI+NNRTI, since these classes of drugs constitute the backbone of first-line therapy in Cuba.
The most frequent mutations found for NRTI and NNRTI in previously treated patients were expected because, for over a decade, AZT+3TC+NVP has been the most common combination used in Cuba for first-line therapy.[12] Worrisome is the high prevalence of V82A mutation which is selected by ritonavir and produces treatment failure with most PI.[27]
In Cuba, HIV-1 patients can only receive ART if it is prescribed by authorized HIV specialists; thus, our observation of NNRTI an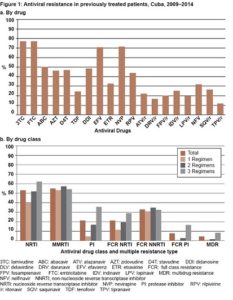 d PI resistance mutations in patients never exposed to these drug classes (Table 2) supports previous reports that drug-resistant strains are in circulation.[11,19] Subtype B viruses played a major role in the earliest ARV resistance studies, most of which reported that existing ARVs are equally effective at treating subtype B and non-B viruses. However, protease and reverse transcriptase sequence data from non-B subtypes isolated from previously treated patients have shown several drug resistance mutations that preferentially occur in certain HIV-1 subtypes. Most of these subtype-specific differences in drug resistance mutation distribution are attributed to differences in codon usage.[28]
d PI resistance mutations in patients never exposed to these drug classes (Table 2) supports previous reports that drug-resistant strains are in circulation.[11,19] Subtype B viruses played a major role in the earliest ARV resistance studies, most of which reported that existing ARVs are equally effective at treating subtype B and non-B viruses. However, protease and reverse transcriptase sequence data from non-B subtypes isolated from previously treated patients have shown several drug resistance mutations that preferentially occur in certain HIV-1 subtypes. Most of these subtype-specific differences in drug resistance mutation distribution are attributed to differences in codon usage.[28]
ART susceptibility of different HIV-1 subtypes is currently the subject of much attention and hence, further research on this topic is encouraged. Our finding that K65R resistance mutation was more likely detected in subtype C is consistent with previous reports.[29–32] The higher prevalence of NRTI mutation L210W in the viral strain CRF19_cpx, has important implications for NRTI-based ART regimens in Cuba, because CRF19_cpx is the third most frequent strain in the Cuban HIV-1 epidemic,[11–13] and has recently been associated with rapid progression to AIDS.[33] Moreover, the higher prevalence of PI mutation M47V/I among Cuban recombinants represents a hazard for PI-based ART.[34] CRF19_cpx and CRFs BGs circulate almost exclusively in Cuba,[11–13] so there are no previous prevalence studies of resistance mutations among these CRFs. Further studies are required to confirm our findings.
Overall, drug resistance to NRTI, NNRTI and PI in the sample studied is high, probably due to the combination’s lack of potency, acquisition of resistant virus[12,35,36] and lower frequency of viral load testing. Despite overall high resistance, our analysis showed a significantly declining trend over time for FCR NRTI, FCR PI and MDR. This might be due to changes in patient selection for resistance testing. In the first years after implementation of the test, samples were selected mainly from patients failing multiple therapy regimens; after 2011, all patients failing first therapy regimen were tested. It might also reflect b etter clinical management of HIV ART, greater experience of clinicians, and virologists’ assistance in interpreting genotypic resistance assays, resulting in increasing ART effectiveness.[8] The declining resistance observed in Cuba is in line with a trend observed in recent years in high-income countries in Western Europe and North America.[35,37,38]
etter clinical management of HIV ART, greater experience of clinicians, and virologists’ assistance in interpreting genotypic resistance assays, resulting in increasing ART effectiveness.[8] The declining resistance observed in Cuba is in line with a trend observed in recent years in high-income countries in Western Europe and North America.[35,37,38]
The study’s main limitation is that it does not meet WHO standards for a national surveillance study, which require a nationally representative sample.[39]
CONCLUSIONS
TDR levels observed reinforce the need for a national surveillance study of Cuban treatment-naïve patients. Despite the high prevalence of resistance in patients failing ART, its frequency seems to be decreasing over time. The frequency of specific drug resistance mutations in recombinant forms of HIV in Cuba needs further attention.
ACKNOWLEDGMENTS
The authors thank Dr Anne-Mieke Vandamme and Kristel Van Laethem for expert advice on design and implementation of RT-PCR and sequencing protocols, and for their contributions and support to building laboratory personnel capacity.
References

- Bertagnolio S, Beanland RL, Jordan MR, Doherty M, Hirnschall AG. The World Health Organization’s response to emerging human Immunodeficiency virus drug resistance and a call for global action. J Infect Dis. 2017 Dec 1;216(Suppl 9):S801–S4.
- AIDS DATA 2015 [Internet]. Geneva: UNAIDS; 2016 [cited 2017 Sep 16]. 80 p. Available from: http://www.unaids.org/sites/default/files/media_asset/2016-AIDS-data_en.pdf
- Aragonés C, Sánchez L, Campos JR, Pérez J. Antiretroviral therapy adherence in persons with HIV/AIDS in Cuba. MEDICC Rev [Internet]. 2011 Apr [cited 2016 Oct 5];13(2):17–23. Available from: http://www.medicc.org/medicc
review/pdf.php?lang=en&id=192
- Pérez L, Álvarez LP, Carmona R, Aragonés C, Delgado E, Thomson MM, et al. Genotypic resistance to antiretroviral drugs in patients infected with several HIV type 1 genetic forms in Cuba. AIDS Res Hum Retroviruses [Internet]. 2007 Mar [cited 2017 Sep 9];23(3):407–14. Available from: https://www.liebertpub.com/doi/pdf/10.1089/aid.2006.0155
- Alemán Y, Vinken L, Kourí V, Pérez L, Álvarez A, Abrahantes Y, et al. Performance of an in-house human immunodeficiency virus type 1 genotyping system for assessment of drug resistance in Cuba. PLoS One [Internet]. 2015 [cited 2017 Sep 16];10(2):e0117176. Available from: http://journals.plos.org/plosone/article/file?id=10.1371/journal.pone
.0117176&type=printable
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol [Internet]. 2013 [cited 2017 Sep 19];30(12):2725–9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3840312/pdf/mst197.pdf
- Bennett DE, Camacho RJ, Otelea D, Kuritzkes DR, Fleury H, Kiuchi M, et al. Drug resistance mutations for surveillance of transmitted HIV-1 drug-resistance: 2009 update. PLoS One [Internet]. 2009 [cited 2017 Sep 16];4(3):e4724. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2648874/pdf/pone.00047
pdf
- Vercauteren J, Deforche K, Theys K, Debruyne M, Duque LM, Peres S, et al. The incidence of multidrug and full class resistance in HIV-1 in fected patients is decreasing over time (2001-2006) in Portugal. Retrovirology [Internet]. 2008 Feb 1 [cited 2017 Sep 16];5:12. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2265747/pdf/1742-4690-5-12.pdf
- Hervada Vidal X, Santiago Pérez MI, Vázquez Fernández E, Castillo Salgado C, Loyola Elizondo E, Silva Ayçaguer LC. Epidat 3.0: programa para análisis epidemiológico de datos tabulados. Rev Esp Salud Pública [Internet]. 2004 [cited 2017 Sep 16];78(2):277–80. Available from: https://scielosp.org/pdf/resp/2004.v78n2/277-280/es. Spanish.
- World Medical Association. Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA [Internet]. 2013 Nov 27 [cited 2018 Jun 17];310(20):2191–4. Available from: https://jamanetwork.com/jour
nals/jama/fullarticle/10.1001/jama.2013.281053
- Pérez L, Kourí V, Alemán Y, Abrahantes Y, Correa C, Aragonés C, et al. Antiretroviral drug resistance in HIV-1 therapy-naive patients in Cuba. Infect Genet Evol [Internet]. 2013 Jun [cited 2017 Sep 16];16:144–50. Available from: http://www.sciencedirect.com/science/article/pii/S1567134813000361?via%3Dihub
- Kourí V, Alemán Y, Pérez L, Pérez J, Fonseca C, Correa C, et al. High frequency of antiviral drug resistance and non-B subtypes in HIV-1 patients failing antiviral therapy in Cuba. J Clinical Virol [Internet]. 2012 Dec [cited 2017 Sep 16];55(4):348–55. Available from: http://www.sciencedirect.com/science/article/pii/S138665321200323X?via%3Dihub
- Machado LY, Blanco M, Dubed M, Díaz HM, Ruiz NM, Váldes N, et al. HIV type 1 genetic diversity in newly diagnosed Cuban patients. AIDS Res Hum Retroviruses [Internet]. 2012 Aug [cited 2017 Sep 18];28(8):956–60. Available from: http://online.liebertpub.com/doi/full/10.1089/2011.0295
- Pérez L, Thomson MM, Bleda MJ, Aragonés C, González Z, Pérez J, et al. HIV Type 1 molecular epidemiology in Cuba: high genetic diversity, frequent mosaicism, and recent expansion of BG intersubtype recombinant forms. AIDS Res Hum Retroviruses [Internet]. 2006 Aug [cited 2017 Sep 18];22(8):724–33. Available from: http://online.liebertpub.com/doi/pdfplus/10.1089/aid.2006.22.724
- Nadai Y, Eyzaguirre LM, Sill A, Cleghorn F, Nolte C, Charurat M, et al. HIV-1 epidemic in the Caribbean is dominated by subtype B. PLoS One [Internet]. 2009 Mar [cited 2017 Sep 18];4(3):e4814. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2652827/pdf/pone.0004814.pdf
- Vaughan HE, Cane P, Pillay D, Tedder RS. Characterization of HIV type 1 clades in the Caribbean using pol gene sequences. AIDS Res Hum Retroviruses [Internet]. 2003 Oct [cited 2017 Sep 18];19(10):929–32. Available from: http://online.liebertpub.com/doi/pdfplus/1089/088922203322493120
- Thomson MM, Casado G, Posada D, Sierra M, Nájera R. Identification of a novel HIV-1 complex circulating recombinant form (CRF18_cpx) of Central African origin in Cuba. AIDS (London, England). 2005 Jul 22;19(11):1155–63.
- Casado G, Thomson MM, Sierra M, Nájera R. Identification of a novel HIV-1 circulating ADG intersubtype recombinant form (CRF19_cpx) in Cuba. J Acquir Immune Defic Syndr. 2005 Dec 15;40(5):532–7.
- Machado LY, Dubed M, Díaz H, Ruiz N, Romay D, Valdés N, et al. Transmitted HIV type 1 drug resistance in newly diagnosed Cuban patients. AIDS Res Hum Retroviruses [Internet]. 2013 Feb [cited 2017 Sep 18];29(2):411–4. Available from: http://online.liebertpub.com/doi/full/10.1089/aid.2012.0183
- Myers JE, Taylor BS, Rojas Fermín RA, Reyes EV, Vaughan C, José L, et al. Transmitted drug resistance among antiretroviral-naive patients with established HIV type 1 infection in Santo Domingo, Dominican Republic and review of the Latin American and Caribbean literature. AIDS Res Hum Retroviruses [Internet]. 2012 Jul;28(7):667–74. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3380383/pdf/aid.2010.0355.pdf
- Barrow GJ, Hylton-Kong T, Rodríguez N, Yamamura Y, Figueroa JP. HIV-1 drug resistance in treatment-naive, chronically infected patients in Jamaica. Antivir Ther [Internet]. 2013 [cited 2017 Sep 21];18(7):941–4. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4648998/pdf/nihms733672.pdf
- Escoto-Delgadillo M, Torres-Mendoza BM, Flores-Soto M, Vázquez-Valls E. HIV drug resistance in antiretroviral-naive patients in Mexico after 10 years: is there a difference? AIDS Res Hum Retroviruses. 2016 Dec;22(12):1219–22.
- Ávila-Ríos S, García-Morales C, Garrido-Rodríguez D, Tapia-Trejo D, Girón-Callejas AC, Mendizábal-Burastero R, et al. HIV-1 drug resistance surveillance in antiretroviral treatment-naive individuals from a reference hospital in Guatemala, 2010-2013. AIDS Res Hum Retroviruses [Internet]. 2015 Apr [cited 2017 Sep 21];31(4):401–11. Available from: http://online.liebertpub.com/doi/10.1089 /aid.2014.0057
- Holguín A, Yebra G, Martín L, de Pineda AT, Ruiz LE, Quezada AY, et al. Transmitted drug-resistance in human immunodeficiency virus-infected adult population in El Salvador, Central America. Clin Microbiol Infect [Internet]. 2013 Dec [cited 2017 Sep 21];19(12):E523–32. Available from: http://www.sciencedirect.com/science/article/pii/S1198743X14630913?via%3Dihub
- Ávila-Ríos S, García-Morales C, Tapia-Trejo D, Meza RI, Nuñez SM, Parham L, et al. HIV drug resistance surveillance in Honduras after a decade of widespread antiretroviral therapy. PLoS One [Internet]. 2015 [cited 2017 Sep 21];10(11):e0142604. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4641727/pdf/pone.0142604.pdf
- Mendoza Y, Castillo Mewa J, Martínez AA, Zaldívar Y, Sosa N, Arteaga G, et al. HIV-1 antiretroviral drug resistance mutations in treatment naive and experienced Panamanian subjects: impact on national use of EFV-based schemes. PLoS One [Internet]. 2016 Apr 27 [cited 2017 Sep 21];11(4):e0154317.Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4847863/pdf/pone.0154317.pdf
- Jones LR, Moretti F, Calvo AY, Dilernia DA, Manrique JM, Gómez-Carrillo M, et al. Drug resistance mutations in HIV pol sequences from Argentinean patients under antiretroviral treatment: subtype, gender, and age issues. AIDS Res Hum Retroviruses [Internet]. 2012 Aug [cited 2017 Sep 26];28(8):949–55. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3399568/pdf/aid.2011.0287.pdf
- Clutter DS, Jordan MR, Bertagnolio S, Shafer RW. HIV-1 drug resistance and resistance testing. Infect Genet Evol [Internet]. 2016 Dec [cited 2017 Sep 29];46:292–07. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5136505/pdf/nihms817643.pdf
- Doualla-Bell F, Avalos A, Brenner B, Gaolathe T, Mine M, Gaseitsiwe S, et al. High prevalence of the K65R mutation in human immunodeficiency virus type 1 subtype C isolates from infected patients in Botswana treated with didanosine-based regimens. Antimicrob Agents Chemother [Internet]. 2006 Dec [cited 2017 Sep 26];50(12):4182–5. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1693987/pdf/0714-06.pdf
- Doualla-Bell F, Avalos A, Gaolathe T, Mine M, Gaseitsiwe S, Ndwapi N, et al. Impact of human immunodeficiency virus type 1 subtype C on drug resistance mutations in patients from Botswana failing a nelfinavir-containing regimen. Antimicrob Agents Chemother [Internet]. 2006 Jun [cited 2017 Sep 26];50(6):2210–3. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1479146/pdf/1447-05.pdf
- Coutsinos D, Invernizzi CF, Xu H, Moisi D, Oliveira M, Brenner BG, et al. Template usage is responsible for the preferential acquisition of the K65R reverse transcriptase mutation in subtype C variants of human immunodeficiency virus type 1. J Virol [Internet]. 2009 Feb [cited 2017 Sep 26];83(4):2029–33. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2643749/pdf/1349-08.pdf
- World Health Organization. WHO Expert Committee on biological standardization. World Health Organ Tech Rep Ser. 2011(962):1–206.
- Kourí V, Khouri R, Alemán Y, Abrahantes Y, Vercauteren J, Pineda-Pena AC, et al. CRF19_cpx is an evolutionary fit HIV-1 variant strongly associated with rapid progression to AIDS in Cuba. EBioMedicine [Internet]. 2015 Jan 28 [cited 2017 Sep27];2(3):244–54. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4484819/pdf/main.pdf
- Wensing AM, Calvez V, Günthard HF, Johnson VA, Paredes R, Pillay D, et al. 2015 Update of the Drug Resistance Mutations in HIV-1. Top Antivir Med [Internet]. 2015 [cited 2017 Sep 27];23(4):132–41. Available from: http://www
.iasusa.org/sites/default/files/tam/23-4-132.pdf
- Vercauteren J, Theys K, Carvalho AP, Valadas E, Duque LM, Teofilo E, et al. The demise of multidrug-resistant HIV-1: the national time trend in Portugal. J Antimicrob Chemother [Internet]. 2013 [cited 2017 Sep 29];68(4):911–4. Available from:https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3594492/pdf/dks470.pdf
- Vandamme AM, Camacho RJ, Ceccherini-Silberstein F, de Luca A, Palmisano L, Paraskevis D, et al. European recommendations for the clinical use of HIV drug resistance testing: 2011 update. AIDS Rev [Internet]. 2011 Apr–Jun [cited 2017 Sep 27];13(2):77–108. Available from: http://www.aidsreviews.com/get
.php?x=2011_13_2_077-108.pdf&dp=0
- Franzetti M, Violin M, Antinori A, De Luca A, Ceccherini-Silberstein F, Gianotti N, et al. Trends and correlates of HIV-1 resistance among subjects failing an antiretroviral treatment over the 2003-2012 decade in Italy. BMC Infect Dis [Internet]. 2014 Jul 18 [cited 2017 Sep 27];14:398. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4223427/pdf/1471-2334-14-398.pdf
- Schmidt D, Kollan C, Fätkenheuer G, Schülter E, Stellbrink HJ, Noah C, et al. Estimating trends in the proportion of transmitted and acquired HIV drug resistance in a long term observational cohort in Germany. PLoS One [Internet]. 2014 Aug 22 [cited 2017 Sep 27];9(8):e104474. Available from: http://journals.plos.org/plosone/article/file?id=10.1371/journal.pone.0104
474&type=printable
- World Health Organization. Surveillance of HIV drug resistance in adults receiving ART (acquired HIV drug resistance). Concept Note [Internet]. Geneva: World Health Organization; 2014 [cited 2017 sept 16]. 50 p. Available from:www.who.int/hiv/pub/drugresistance/acquired_drugresistance/en/
THE AUTHORS
Yoan Alemán-Campos,* biochemist with a master’s degree in virology. Assistant professor and adjunct researcher, Virology Department, Pedro Kourí Tropical Medicine Institute (IPK), Havana, Cuba.
Vivian Kourí-Cardellá (Corresponding author: vkouri@ipk.sld.cu),* physician specializing in microbiology, with a master’s degree in virology and infectious diseases, a doctorate in virology and an advanced doctorate. Full professor and senior researcher, Virology Department, IPK, Havana, Cuba.
Lissette Pérez-Santos,* microbiologist with a master’s degree in virology and doctorate in health sciences (virology). Associate professor and senior researcher, Virology Department, IPK, Havana, Cuba.
Carlos Fonseca-Gómez, internist with a master’s degree in infectious diseases. Associate professor, Clinical Department, IPK, Havana, Cuba.
Jorge Pérez-Avila, physician specializing in pharmacology with a master’s degree in clinical pharmacology. Associate professor and senior researcher, Clinical Department, IPK, Havana, Cuba.
Lilia M. Ortega-González, physician with dual specialties in internal medicine and intensive care, with a master’s degree in infectious diseases. Associate professor and researcher, Clinical Department, IPK, Havana, Cuba.
Yoanna Baños-Morales, chemistry technician, Virology Department, IPK, Havana, Cuba.
Alina Álvarez-López, chemistry technician, Virology Department, IPK Havana, Cuba.
Consuelo Correa-Sierra, physician specializing in microbiology, with a master’s degree in virology and a doctorate in medical sciences, Virology Department, IPK Havana, Cuba.
Yenisleidys Martínez-Montesinos, microbiologist with a master’s degree in virology, Virology Department, IPK, Havana, Cuba.
Yudira Soto-Brito, microbiologist with a master’s degree in virology and a doctorate in health sciences (virology). Associate professor and senior researcher, Virology Department, IPK, Havana, Cuba.
Celia M. Limia-León, biochemist with a ma-ster’s degree in virology, Virology Department, IPK, Havana, Cuba.
Jorge Campos-Díaz, informatics technician, Informatics Department, IPK, Havana, Cuba.
Yaniris Caturla-Fernández, technician, Clinical and Microbiology Laboratory, Virology Department, IPK, Havana, Cuba.
Delmis Alvarez-Gainza, computer scientist with a master’s degree in epidemiology, Informatics Department. IPK, Havana, Cuba.
Yanet Pintos-Saavedra, microbiologist, Viro-logy Department, IPK, Havana, Cuba.
Ana L. Añé-Kourí, physician specializing in clinical biochemistry. Virology Department, IPK, Havana, Cuba.
José Joanes-Fiol, physician specializing in epidemiology, with a master’s degree in pu-blic health, HIV/STI Program, Ministry of Public Health, Havana, Cuba.
*The first three authors contributed equally to this research
Submitted: October 27, 2017 Approved: July 01, 2018 Disclosures: This work was supported by grants from the Vlaamse Interuniversitaire Raad (ZEIN2008PR358); the Global Fund to Fight AIDS, Tuberculosis and Malaria; and Cuba’s Ministry of Public Health. The authors declare no competing interests.









