INTRODUCTION
Infections HIV-2 and HIV-1 cause acquired immunodeficiency syndrome (AIDS). Although clinical symptoms are similar in both cases, HIV-2 is less pathogenic; the latency period is longer than ten years; and case-fatality is estimated to be one half to one third that of HIV-1, possibly due to lower viral load.[1,2]
Globally, most HIV infections are caused by HIV-1. However, HIV-2, initially restricted to West Africa,[3] has spread to all continents.[4–6] Most cases diagnosed outside western African countries are epidemiologically linked to persons in that region or native to it and have been found mainly in Portugal, France, Spain and southeast India.[7–10] Less frequently, cases have been reported in other European countries and in the Americas and Asia.[11–13]
HIV-1 and HIV-2 infections differ in prognosis and require different treatment strategies; thus, differential diagnosis is the initial step for better understanding transmission, epidemiology and pathogenesis of HIV infection in geographical areas where both viruses circulate.[6,11]
Spread of HIV-2 has led to development of specific diagnostic systems. In 1990, the US Food and Drug Administration certified the first assay for HIV-2 diagnosis and in 1991, the first combined HIV-1/2 system,[13] enabling simultaneous antibody screening for both retroviruses.
However, high structural homology between HIV-1 and HIV-2 genomic products, mainly in gag and pol gene derived proteins, causes serological cross reactions in diagnostic systems for both viruses, preventing definitive results.[13] This problem led to development of enzyme-linked immunosorbent assays (ELISA), with elevated sensitivity and specificity due to their ability to distinguish each virus’ specific reactivity by using synthetic antigens that mimic specific immunodominant epitopes of the most immunogenic and persistent proteins.[13,14]
Both these viruses circulate in Cuba.[15–17] According to the National HIV/AIDS Program (full title: National Program for Prevention and Control of Sexually-Transmitted Diseases and HIV/AIDS), the first HIV-2 case was diagnosed in 1987 and through December 2004, 16 cases had been confirmed; three of the patients now deceased (National Epidemiology Division, Ministry of Public Health, unpublished data).
The HIV-1/2 diagnostic algorithm in Cuba is aimed at sensitive and specific identification of infection at low cost, and coincides with those of developed countries, such as USA, France and Canada.[18–21] In a first stage, samples are analyzed with HIV-1/2 mixed screening techniques with ultramicroanalytic ELISA and ELISA, confirming reactive samples with HIV-1 Western blot (WB). HIV-2 infection is among the possible causes of indeterminate WB results, the test’s main limitation; hence, the importance of second-stage screening and confirmatory tests to diagnose HIV-2 infection in reactive samples with negative or indeterminate results by HIV-1 WB.
The objective of this study was to investigate the contribution of HIV-2 antibodies to negative or indeterminate HIV-1 WB results in serum samples taken during the 2005–2008 period.
METHODS
This is a prospective screening study.
Diagnostic algorithm HIV-1/2 infection diagnosis in Cuba (Figure 1) follows the diagnostic strategy proposed by the WHO for countries with low prevalence of HIV-1/2 infection.[18,22]
Figure 1: Diagnostic algorithm for Cuba’s National HIV/AIDS Program

RR: Repeatedly reactive WB: Western Blot RNR: Repeatedly nonreactive LNR: National Retrovirus Reference Laboratory SUMA: Ultramicroanalytic system, screening technology developed in Cuba * If indeterminate, follow up with infectious disease consultation and assess antigen detection with PCR or culture.
Source: Diaz H et al.[18] Adapted with permission.
Initial HIV-1/2 antibody screening is done with the ultramicroanalytic system UMELISA HIV 1+2 recombinant (TecnoSuma, Cuba). Repeatedly reactive samples are sent to the AIDS Research Laboratory (LISIDA, its Spanish acronym), in which a second HIV-1/2 antibody screening is performed using a commercial HIV-1/2 ELISA (Vironostika Uni-Form II Ag/Ab for HIV-1 and -2, bioMérieux, Netherlands).
Reactive samples are confirmed by HIV-1 WB (DAVIH–blot, DAVIH Laboratories, Cuba). HIV-1 WB-indeterminate or -negative samples are analyzed with the HIV-2 ELISA, DAVIH–VIH-2 (DAVIH Laboratories, Cuba); samples that are nonreactive by ELISA that were indeterminate by DAVIH–blot for HIV-1 lead to serological patient followup for at least one year.
Duplicate ELISA-reactive samples are tested with HIV-2 WB- positive results are confirmed by a second sample and the same previously described followup is carried out if results are indeterminate or negative.
Samples A total of 1723 serum samples that were negative (n=295) or indeterminate (n=1428) by HIV-1 WB (DAVIH–blot, DAVIH Laboratories, Cuba) were studied from January 2005 through December 2008. Previously, they had been characterized as HIV-1/2 reactive by UMELISA HIV 1+2 Recombinant (TecnoSuma, Havana, Cuba) and Vironostika Uni-Form II Ag/Ab for HIV-1 and -2 (bioMérieux, Netherlands).
Serological tests for HIV-2 diagnosis The ELISA DAVIH–VIH-2 system (DAVIH Laboratories, Cuba) is an indirect ELISA for HIV-2 antibody screening in human plasma or serum, its solid phase presenting a synthetic peptide with a 20 amino-acid sequence of the 36 kD protein (gp36) of this virus. In its validation stage, the test showed sensitivity and specificity values of 100% and 98%, respectively.[16] To interpret results, sample optical density values were standardized according to the instruction manual of the ELISA DAVIH–VIH-2 system. Samples with standardized values ≥0.0365 were considered HIV-2 antibody reactive and those with values of <0.365 were considered nonreactive by ELISA.
HIV-2 Western blot Natural HIV-2 antigen was obtained from viral culture and applied on 11% SDS-polyacrylamide gel, to separate viral proteins.[23] These proteins were transferred to 0.2 µm nitrocellulose membrane (Bio-Rad Laboratories, USA) using a BioRad transference chamber (Bio-Rad Laboratories, USA), during 2 hours at 4 °C, as described by Towbin and colleagues.[24] Sensitized membranes were washed with distilled water, dried with filter paper and cut in 0.3 cm strips. They were numbered and stored at 4 °C until use. The diluted serum sample (1:100 in sample dilution buffer plus 5% skimmed milk) was added to sensitized strips and incubated for 18 to 20 hours. After incubation and washings, horseradish peroxidase conjugate anti-human sheep IgG (Sigma-Aldrich, USA) diluted 1:1000 in dilution buffer plus 5% skimmed milk was added and incubated for one hour. Strips were developed with substrate buffer solution containing 0.08% hydrogen peroxide and the chromogenic substrate 0.05% diaminobenzidine tetrahydrocloride (Sigma-Aldrich, USA). Finally, the reaction was stopped with distilled water when colored bands appeared (3–5 minutes).
System components for HIV-2 WB were standardized in the DAVIH Laboratories, Cuba, and are undergoing assessment for registration. In evaluations carried out using WHO and LISIDA reference panels, the diagnostic kit showed sensitivity and specificity values of 100% and 80%, respectively (unpublished data).
Criterion for HIV-2 WB interpretation WB assay results were interpreted according to WHO-recommended criteria,[13,14,25] by which a sample is considered positive if at least two of the three bands coded by the env gene (gp36, gp105, gp140) are present, negative if no band appears and indeterminate when there is a band profile that does not classify as positive or negative.
The complete pattern observed in HIV-2–positive WB includes a total of nine structural bands corresponding to proteins (p) and glycoproteins (gp) coded by the gag, pol and env virus genes: p16(gag), p26(gag), gp36(env), p53(pol), p56(gag), p68(pol), gp105(env) and gp140(env).[13,14]
Ethical aspects The study design and ethics were reviewed and approved by LISIDA’s scientific council. Following informed consent, an epidemiologic interview established by Cuba’s National HIV/AIDS Program was administered to patients with HIV-2 WB-positive samples. In addition to general data, it gleans information on potential contagion sources and patients’ sexual contacts.[26] All tests were carried out for clinical management of the patients under study and measures were taken to ensure patient anonymity.
RESULTS
DAVIH–VIH-2 ELISA identified 12 reactive samples (0.70%) and 1711 nonreactive ones (99.30%) in the total number of sera studied from HIV-1 WB-negative or -indeterminate persons during a four-year period (Table 1).
Table 1: HIV‑2 antibody detection by year and type of assay
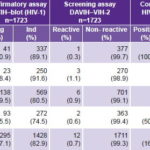
n: Number of samples Neg: Negative result Ind: Indeterminate result
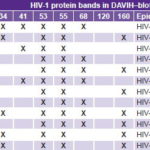
Table 2: Antibody pattern against HIV‑1 WB protein bands in 12 HIV‑2 ELISA‑reactive samples
Analysis of these 12 ELISA-reactive samples using HIV-2 WB confirmed 2 positive samples 16.7%), 4 negative (33.3%) and 6 indeterminate (50%) (Table 1).
Samples complied with the minimum-level criterion of positivity displaying reactivity against two of the envgene proteins (gp36 and gp105); as well, they showed reactivity against the remaining gag (p16, p26, p56) and pol (p68, p53) gene proteins (Figure 2). The 12 HIV-2 ELISA-reactive sera were confirmed as belonging to the HIV-1 WB-indeterminate group (Table 2). The two HIV-2 positive samples exhibited well-defined reactivity against gp160, p53, p55 and p34 of HIV-1.
In serologic followup of patients providing the remaining 10 samples, HIV-1 seroconversion was observed in all.
DISCUSSION
DAVIH–VIH-2 classified more than 99% of the analyzed samples as non-reactive. Consistency of these results is reinforced by the high negative predictive value of the system (100%), given sensitivity and specificity values observed in validation [16] and the low HIV-2 prevalence in Cuba.[15,16] These results are consistent with those of Martín et al. in 2007, evaluating a system with samples of similar characteristics.[16]
Patients with HIV-1 WB-indeterminate results constitute one of the groups given most attention from a diagnostic point of view; more so if they have a previous positive result using HIV-1/2 mixed screening systems. These results constitute the WB resolution limit and include band patterns that do not fulfill the minimum criterion of positivity; moreover, indeterminate results are a constant source of concern for both patients and staff performing the assay.[27,28] Among its causes are factors not associated with HIV infection: presence of autoantibodies such as rheumatoid factor, antibodies against human lymphocyte antigens (HLA), alloimmunization by transfusions or transplants, presence of contaminating cell antigens in the viral lysates used to prepare WB strips, pregnancy, infection by other retroviruses such as HTLV-I/II, elevated bilirubin levels, “in vitro” hemolysis, heterophile antibodies against animal proteins used in the WB assay, and antibodies in individuals with parasitic or other viral infections. Causes associated with HIV-1 infection include antibody production against viral nuclear antigens during early infection or loss of antibodies in terminal stages, infection by simian immunodeficiency virus and HIV-2 infection.[27–29] In the present study, HIV-2 infection was one of the causes, although not the most frequent one; its effect is likely due to high structural homology among proteins derived from the gag, pol and even env genes of HIV-1 and -2, which causes serological cross reactions in diagnostic systems for the two viruses.[6,13,30]
The Cuban diagnostic algorithm involves serological followup of individuals with indetersminate results for at least a year to define their status; they may seroconvert to positivity, test negative, or remain indeterminate.[18] Although it is true that these later studies indicate that most indeterminate results do not seroconvert,[31] it is noteworthy that this depends on the person’s epidemiological risk, clinical status and serologic profile.[13] Nucleic acid amplification assays in HIV diagnostic algorithms are useful in resolving indeterminate WB results, but their high cost impedes their use in studying large numbers of samples and in financially-constrained systems.[32] In Cuba this type of test is mainly used in pregnant women who refer to sexual contact with seropositive persons, in followup of children through 18 months of age born to seropositive mothers, and in individuals with clinical and/or epidemiological suspicion of infection who maintain an indeterminate WB pattern.[18,28]
Figure 2: Reactivity pattern for samples positive for HIV 2 by Western blot
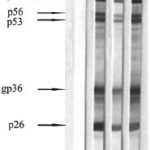
1. Negative control 2. HIV 2 positive control 3. Sample 11 4. Sample 12
The HIV-1 WB-indeterminate patterns of the two HIV-2 positive samples are consistent with reports by other authors who have analyzed HIV-2 positive samples by HIV-1 WB, which mainly display reactivity against gag (p55) and pol (p34, p53) antigens.[30,33,34] Reactivity against gp 160 is less frequent and is observed in B subtypes of HIV-2.[30] Followup of the remaining persons later diagnosed with HIV-2 in Cuba found only one more reactive to gp160 of HIV-1 (Díaz D, unpublished data).
HIV-2 ELISA-reactive results of the 10 samples that were not positive by HIV-2 WB may be explained by serological cross reactions due to similarity between structural proteins of these viruses.[6,13,30] Moreover, these samples’ optical density values in HIV-2 ELISA were lower than those shown by the two positive samples and the positive control on the plate. Similar results have been reported by other authors evaluating specificity of HIV-2 ELISA systems with HIV-1 positive sera.[16,34,35]
The results showed the usefulness of DAVIH–VIH-2 for screening in the HIV-1/2 diagnostic algorithm in Cuba, where a low prevalence of HIV-2 infection has been demonstrated.[15,16] The two newly-confirmed cases in this study—one diagnosed in 2005 and one in 2008—bring the number of HIV-2 seropositive persons in Cuba to 18. Both patients were female, heterosexual, aged 36 and 20 years, and residents of Villa Clara and the City of Havana, respectively—the provinces with the highest numbers of HIV-2 cases in Cuba. It was not possible to determine the probable source of infection in epidemiological interviews.
The combined use of ELISA and WB enabled discrimination between infections by HIV-1 and HIV-2 and avoiding overestimation of HIV-2 infection. This is very important for both epidemiologic surveillance and clinical management, due to differences in pathogenesis and treatment.
In this study, an internationally-marketed system was not used to confirm HIV-2 ELISA-reactive samples, which may represent a limitation. However, the system used showed high sensitivity and specificity values in prior evaluations.
Our results lead us to recommend that HIV-1 WB-indeterminate and -negative cases be followed up with a combination of HIV-2 ELISA and WB. Confirmatory testing should of course employ a system registered and approved by national public health authorities (in process for the system described here).
CONCLUSIONS
Two new cases of HIV-2 seropositivity were diagnosed using DAVIH–VIH-2 and HIV-2 WB in HIV-1 WB–indeterminate samples. The HIV-2 WB system should be incorporated in LISIDA’s HIV-1/2 diagnostic algorithm.
ACKNOWLEDGMENTS
The authors acknowledge Dr Hector M. Díaz Torres and Dr Otto Cruz Sui for advice and comments during writing of this paper.







