INTRODUCTION
The female genital system is colonized by microorganisms of bacterial origin. When imbalance of the vaginal microbiota occurs, aerobic and anaerobic bacteria multiply, causing disease.[1] Pathogens such as Neisseria gonorrhoeae and Chlamydia trachomatis also penetrate the female genital system through unprotected sex.[2] Mycoplasma hominis, Ureaplasma urealyticum and Mycoplasma genitalium are the mollicutes (class of bacteria lacking cell walls) most frequently isolated in the genital tract and the most potentially pathogenic. They are associated with pelvic inflammatory disease, urethritis, salpingitis, bacterial vaginosis, infertility, ectopic pregnancy, obstetric pathologies (spontaneous abortion, preterm delivery and puerperal infections) as well as perinatal disorders (low birth weight, respiratory and neurological infections).[3,4]
International research suggests an increase in genital mycoplasma infections over the last decade.[1,3] Prevalence reported internationally varies: 3.9%–31% in Mexico,[5-7] 44.8% in China[8] and 54.9% in Turkey.[9] In Cuba, prevalence in patients with vaginal discharge is unknown. Their biochemical properties require special culture media; hence, their isolation and identification by conventional bacteriology is difficult.[10,11] Rapid diagnostic tests for urogenital mycoplasmas are a useful alternative where lack of resources do not permit use of conventional isolation techniques.[10,12]
As with all pathogenic microorganisms, knowing vaginal mycoplasma antimicrobial sensitivity allows the physician to select the appropriate antibiotic for acute infections, and thus avoid chronic sequelae and microbial resistance.[3,8]
Globally, vaginal discharge is a frequent reason for medical consultation.[1,3,11] The objective of this study was to determine frequency and antimicrobial sensitivity of U. urealyticum and M. hominis in patients with vaginal discharge receiving attention at the Municipal Hygiene and Epidemiology Center in Güines, Mayabeque Province, Cuba.
METHODS
Type of study and patients A descriptive retrospective study based on laboratory data was carried out in the Clinical Microbiology Laboratory of the Municipal Hygiene and Epidemiology Center in Güines municipality. Data pertained to 255 women with vaginal discharge syndrome (increase in amount of vaginal secretions, change in color and consistency, disagreeable odor and vulvar irritation) without prior microbiological study, who were referred to the Laboratory by gynecologists from May 2009 through December 2010 for a diagnostic vaginal smear for U. urealyticum and M. hominis diagnosis.
Sampling and mycoplasma identification The Mycoplasma System Plus (MSP, for detection, identification, count and sensitivity testing of urogenital mycoplasmas and ureaplasmas; Liofilchem SRL, Italy) was used to identify U. urealyticum and M. hominis. Results obtained with this system agree with those of traditional culture methods: the Wilcoxon ranked-sum test was used to compare MSP results with standard values, showing no significant difference, p ≥0.05.[13] Sample collection and processing was carried out according to manufacturer’s instructions. No other laboratory procedure was used to rule out presence of other microorganisms.
Study variable Antimicrobial sensitivity is defined as the in vitro activity of an antibiotic against a specific microorganism and reflects its capacity to inhibit growth of a bacteria or bacterial population. Level of antimicrobial sensitivity was determined by MSP criteria; color changes in the test wells correspond to levels of sensitivity: yellow = sensitivity, orange = intermediate sensitivity and red = resistance.[13]
Determination of antibiotic sensitivity MSP provides integrated sensitivity testing for the following antimicrobial drugs in two concentrations each: tetracycline, 4 and 8 µg/mL; pefloxacin, 8 and 16 µg/mL; ofloxacin, 1 and 4 µg/mL; doxycycline, 4 and 8 µg/mL; erythromycin, 8 and 16 µg/mL; clarithromycin, 8 and 16 µg/mL; minocycline, 4 and 8 µg/mL; clindamycin, 4 and 8 µg/mL and azithromycin, 4 and 8 µg/mL.[13]
Data analysis The study used administrative data from the laboratory control logbook. A database was created and percentages calculated. Data were organized in tables for analysis.
Ethics Data collection and analysis procedures ensured patient anonymity. The study was approved by the ethics committee of the Güines Municipal Hygiene and Epidemiology Center.
RESULTS
Genital mycoplasmas were detected in 63.1% of specimens (161/255). Among these, U. urealyticum alone was present in 68.9% (111/161), M. hominis alone in 4.3% (7/161) and both in 26.7% (43/161).
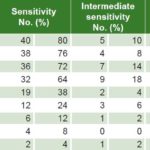
Less than 35% of U. urealyticum specimens showed resistance to doxycycline, pefloxacin, minocycline, clindamycin, tetracycline and azithromycin; resistance to ofloxacin, clarithromycin and erythromycin was seen in 64.3%, 63% and 46.1% respectively. Intermediate sensitivity was observed in 2.6%–9.1% (Table 1). Less than 20% of M. hominis specimens displayed resistance to minocycline, pefloxacin, clindamycin and doxycycline, while resistance to ofloxacin was seen in 70% and clarithromycin, azithromycin and erythromycin in >85%. Intermediate sensitivity was seen in 2%–18% (Table 2). Resistance to ofloxacin was common for both organisms (≥64%), with relatively low proportions (≤18.2%) resistant to minocycline, pefloxacin, clindamycin and doxycycline. M. hominis displayed resistance to more of the antibiotics tested (5/9) than did U. urealyticum (2/9).
Table 1: Antimicrobial sensitivity to Ureaplasma urealyticum (n = 154)
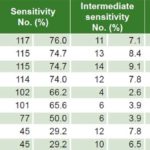
Table 2: Antimicrobial sensitivity to Mycoplasma hominis (n = 50)
DISCUSSION
Laboratory identification of these microorganisms is difficult because they require special broths for transportation and culture. Several agar and broth formulas have been investigated, most difficult to prepare.[14]
Few papers have been published in Cuba on the isolation of vaginal mycoplasma in patients with vaginal discharge. Our results lie between the 34.3% detected by Ortiz in infertile patients and those with repeated abortions,[10] and the 77.5% reported by Fernández in patients with bacterial vaginosis.[11]
U. urealyticum was the predominant species we found, in contrast to results reported by Fernández and by Castellano-González in Venezuela, who found M. hominis in 71% and 35.9% of specimens respectively.[11,15] Our results conflict with those of Agbakoba in Nigeria and González-Pedraza in Cuba, who reported U. urealyticum prevalence of 29.4%[16] and 17.9%,[17] respectively, in symptomatic patients. These discrepancies could be due to differing study populations and detection methods used.
In countries with scarce economic resources, it has been shown that syndromic treatment of sexually transmitted infections is appropriate for high-risk populations and symptomatic patients, whereas in asymptomatic individuals, especially women, risk scores and simple laboratory tests may be required to boost syndromic management algorithm sensitivity.[18,19]
Absence of a cell wall—the target of antimicrobial agents such as penicillins and cephalosporins—confers mycoplasma intrinsic resistance to these beta lactam antibiotics.[20] Increased resistance in vaginal mycoplasmas is reported with global variations to the drugs of choice (tetracycline and doxycycline).[21] Our findings of >65% of U. urealyticum sensitive to tetracyclines and clindamycin, are similar to the results of Solís-Martínez in Mexico[14], Krausse in Germany[21] and Kechagia in Greece.[22] Ortiz in Cuba reported 22% resistance to tetracycline,[10] less than we observed.
Regarding M. hominis antimicrobial sensitivity, Wang in China reported 37.8% resistance to azithromycin and 32.9% to erythromycin,[23] proportions lower than we found, which were >90% to both drugs. Solís-Martínez reported 21.4% of M. hominis resistant to azithromycin, and found it highly sensitive to doxycycline, minocycline and ofloxacin, with resistance of 0%, 7.1% and 14.3%, respectively,[14] in contrast to our results. In Cuba, Ortega also found high resistance to azithromycin.[24]
It is noteworthy that both species studied were resistant to ofloxacin, in contrast to Krausse’s report of >95% sensitivity for both in German populations;[21] our results are more consistent with those of Kechagia in Greece.[22]
For the purposes of this study, isolates with intermediate sensitivity results to any of the antibiotics evaluated were not included in the resistant group, but an antibiotic with high proportions of intermediate sensitivity would not be considered a treatment of choice. Some international authors have treated intermediate sensitivity as resistance.[25,26]
For U. urealyticum, Fagundo (Mexico) found ciprofloxacin resistance most common, followed by ofloxacin; for M. hominis, he most frequently found resistance to the macrolide group (azithromycin, erythromycin, clarithromycin).[25] In Spain, Orellana observed 80.7% of U. urealyticum resistant to ciprofloxacin and 32.4% to ofloxacin, with lower proportions resistant to doxycycline (0.8%) and tetracycline (3.5%).[26] Our results are consistent with Fagundo’s but differ substantially from Orellana’s with respect to ofloxacin resistance.
Although this research involves a short study period and does not include a representative population sample (its main limitations), it is the first study carried out locally in Cuba on this topic. Thus, further research is needed in our province to see whether these results hold for the larger population; such research will require a larger sample size and full access to clinical, epidemiological and microbiological variables.
RECOMMENDATIONS
The finding of mycoplasmas in almost two thirds of specimens examined suggests that the sexually active female population should be screened for them and that barrier contraception methods should be promoted to decrease their spread and prevent longterm sequelae.
Access to updated information about local patterns of antimicrobial resistance supports decision making to determine best treatment options in patients with these infections. MSP enables individualized treatment, but since it is not currently available in all laboratories, clinicians frequently will have to start treatment empirically. Our results should help these clinicians choose an antibiotic, and also confirm the utility of MSP for mycoplasma research in resource-scarce settings, to benefit both individual and population health.





