ABSTRACT
INTRODUCTION Flow cytometry allows immunophenotypic characterization of important lymphocyte subpopulations for diagnosis of diseases such as cancer, autoimmune diseases, immunodeficiencies and some infections. Normal values of rare lymphoid cells in blood, quantified by cytometry, vary among different populations; so it is indispensable to obtain normal national values that can be used in clinical practice.
OBJECTIVE Characterize distribution of rare T-lymphocyte populations in peripheral blood, specifically double-positive T, natural killer T and activated T lymphocytes, as well as their relationship to sex and age.
METHODS A cross-sectional study was carried out in 129 adults (68 women, 61 men) aged >18 years, without chronic diseases or unhealthy habits, who signed informed consent. Peripheral blood was collected for immunophenotyping of lymphocyte subpopulations with monoclonal antibodies specific for CD4+CD8+ double-positive T cells, CD3+CD56+ natural killer T cells, and CD3+CD25+HLA-DR+ activated T cells. An eight-color flow cytometer (Beckman Coulter Gallios) was used. The analytic strategy was modified, associating variables of interest in a single graphic, using conventional monoclonal labeling antibodies. Medians and minimum and maximum percentiles (2.5 and 97.5, respectively) were used as descriptive statistics, stratified by sex, for cell counts and percentages. A linear regression model was applied to assess age effects and a two-tailed Mann-Whitney U test for independent samples was used to assess sex differences. The significance threshold was set as p ≤0.05.
RESULTS Median percentages of total lymphocytes: natural killer T cells 6.3% (1.4%–23%) in men and 4.7% (0.8%–11.3%) in women (p = 0.003); activated T cells 1.0% (0.2%–2.2%) in men and 1.2% (0.4%–3.1%) in women, without statistical significance; and double positives 0.8% (0.1%–4.2%) in men and 0.9% (0.3–5.1) in women, also without statistical significance. Median cell counts (cells/mL) were: natural killer T cells, 126 (27–580) in men and 105 (20–279) in women (p = 0.023); activated T cells: 20 (4–46) in men and 25 (7–75) in women, (p = 0.013) and double-positive T cells: 17 (2–85) in men and 21 (7–154) in women, without statistical significance. Sex influenced natural killer T cells, but age did not.
CONCLUSIONS Age does not affect counts and percentages of rare T lymphocyte subpopulations in the blood of healthy Cuban adults. Sex differences found for some phenotypes suggest the need for different reference values for women and men.
KEYWORDS Normal values, T-lymphocyte subpopulations, flow cytometry, Cuba
INTRODUCTION
Flow cytometry (FC) is a useful technology for analytical and quantitative characterization of cells, because it offers rapid, simultaneous and complete information on distinctive cell aspects. Thus, it has become an essential tool in diagnosis, monitoring and management of numerous diseases, such as immunodeficiencies, infections, autoimmune diseases, cancer and others with immunopathogenic components. Because of its high sensitivity, it is the gold standard for assessing leukemias and natural killer (NK)/T-cell lymphoma, particularly in special biological samples such as cerebrospinal fluid. Its use has extended to other areas of medicine such as post-transplant care.[1–8]
FC has enabled discovery of different lymphocyte populations and shown their great heterogeneity. Previously, the classic lymphocyte populations reported were NK B and T lymphocytes. The latter have two types, TCD4+ and TCD4+8, which once were considered unique and mutually exclusive because of limitations in early cytometry technology that did not allow more than two or three markers on the same cell.[2] We now know that several cell subpopulations form each of these markers (CD4 and CD8). This shows the diversity of lymphocyte subpopulations, each with its own role in immunity; even if rare, they have important functions, so it is essential to consider them for correctly phenotyping peripheral lymphocytes.[6–15]
IMPORTANCE This study is an initial step towards the use of flow cytometry and conventional reagents to quantify rare but important lymphocytes in diagnosis and prognosis of adult Cuban patients with cancer, autoimmune diseases, HIV/AIDS and chronic infections.
The lymphoid cells of innate immunity are an example of recently discovered lymphocyte diversity.[9,10,16–24] These cells are rare in the blood (together <10% of circulating T lymphocytes) but are not necessarily in the minority in other compartments, in lymphoid tissues or elsewhere. They have important functions in maintenance of homeostasis and in immunopathogenesis of some diseases. They are quantified in counts and percentages of total lymphocytes by FC.
T lymphocytes are more heterogeneous than B lymphocytes and some subpopulations can be characterized with the same monoclonal antibodies used for conventional T, B and NK cell protocols, using a different reading window, which implies changing the analytic strategy and associating the variables of interest in a single graphic in the flow cytometer.
Thus far, normal values of these lymphocyte populations in healthy Cuban adults have not been established. Immunophenotyping of rare lymphocytes in peripheral blood extends analysis to more circulating variants, avoiding underreporting of cells with demonstrable clinical importance and not requiring other technologies or additional reagents for their characterization.
The study objective was to characterize the distribution of rare T-lymphocyte populations in peripheral blood and their relation to sex and age in healthy Cuban adults, with the purpose of eventually using them as reference values in diagnosis and prognosis of multiple diseases.
METHODS
Design, subjects and sample collection A cross-sectional study was carried out from January through April 2017 at the Hermanos Ameijeiras Clinical–Surgical Teaching Hospital. We included 129 apparently healthy adults, companions of patients who were seen at the immunology service of the Hermanos Ameijeiras Clinical–Surgical Teaching Hospital, and who provided written informed consent to participate. The sample consisted of 68 women and 61 men, aged 18–80 years (average 40 years). Exclusion criteria were habits, diseases and treatments that could structurally and functionally modify the immune system: toxic habits (smoking, alcohol consumption >40 g daily or its equivalent per week,[25] >4 cups of coffee a day); history of infections; use of antibiotics, immunosuppressants, immunostimulants, anti-inflammatories or anticoagulants in the previous 6 months; diabetes, immunodeficiencies, autoimmune or neoplastic diseases; (for women) pregnancy.
Blood was obtained by peripheral venipuncture; 4 mL were deposited in Vacutainer tubes using ethylene-deamine tetraacetic acid as anticoagulant. Samples were processed within six hours after extraction and the process followed good laboratory practice standards.
Flow cytometry A Beckman Coulter Gallios 8-color cytometer of (Beckman Coulter, France) was used; 100 μL of blood were dispensed for staining with fluorochrome-conjugated monoclonal antibodies from the same manufacturer (Beckman Coulter, France): anti-CD45 AA750 (Clone J33), anti-CD19 (Clone J3-119), anti-CD3 FITC (Clone UCHT1), anti-CD4 PC5.5 (Clone 13B8.2), anti-CD8 AA700 (Clone B9.11), anti-CD56 PE (Clone N901) (NKH-1), anti-HLA-DR PE (Clone Immu-357), anti-CD25 PC5 (Clone B1.49.9). Two panels were designed:
- phenotype of double-positive mature subpopulations and NKT, and
- phenotype of activated T cells.
To characterize the phenotype of T lymphocyte populations, immunophenotyping was done as follows:
- mature double-positive alpha beta T cells (CD45+, CD3+, CD4+CD8+) and their subtypes—
a. CD45+CD3+CD4highCD8low
b. CD45+CD3+CD4lowCD8high
2. NKT cells (CD45+CD3+CD56+) and subtypes—
a. CD45+CD3+CD56+CD4+CD8−
b. CD45+CD3+CD56+CD4−CD8+
c. CD45+CD3+CD56+CD4−CD8−
3. activated T cells—
a. CD45+CD3+HLA-DR+
b. CD45+CD3+CD25+
c. CD45+ CD3+HLA-DR+CD25+
Sample preparation was carried out according to manufacturer’s specifications for cell surface immunophenotyping, using a protocol of unwashed red blood cells with Versalyse buffer (Beckman Coulter, France). Cytometer quality control was performed daily with Flow-Check fluorospheres (Beckman Coulter, France) to align lasers and check the water system. Fluorescence intensity was controlled with Flow-Set fluorospheres from the same company.
Acquisition data were processed using Kaluza Analysis Software V1.5A, with a minimum of 50,000 acquired events, which refers to the number of formed elements contained in a blood sample that pass through the cytometer’s laser light beam and are counted and analyzed. In each case >4000 events were obtained in the lymphocyte region characterized by high expression of CD45 and low complexity as indicated by side scatter. Rare subpopulations were identified with modification of the analysis strategy to associate the variables of interest in a single graphic, permitting analysis of six monoclonal antibodies specific to six cell surface antigens at once, using a single 50-µL blood sample. This is illustrated in Figure 1, where each dot plot represents two parameters analyzed by the cytometer.
Bidimensional analyses were concatenated hierarchically to enable multiparametric analysis and characterization of several cell population phenotypes in a single tube. Thus, the first dot plot in Figure 1a reflects a window through which single cells passed through the laser beam for cell-by-cell analysis. Next a second dot plot was generated to relate cell size to complexity, distinguishing cells from artifacts or detritus. Once cell events were selected, a third dot plot was created that identified groups of leukocytes based on expression of a pan-leukocyte antigen, CD45. The lymphocyte population for analysis is circled at the bottom of the third dot plot. Then antigens representative of each strain were combined successively, from general to specific, each population displayed in a separate quadrant (Figure 1).
Absolute counts were obtained by double platform; results obtained were combined in an automatic hematological counter and by cytometry. The following formula was applied:
Absolute count (cells/μL) = lymphocyte count (number of cells/μL in blood count) x % of the cellular subpopulation of interest ÷ 100.
Analysis Descriptive statistics were calculated: absolute and relative frequencies, means, medians and SD. The Kolmogorov–Smirnov test was used to assess normality of distribution of variable values. Because of variable asymmetry, percentiles 2.5 and 97.5 were specified as lower and upper limits, respectively, for reference intervals. A linear regression model was used to assess the effect of age and the two-tailed Mann–Whitney U test for independent samples to measure the effect of sex. The significance threshold was set at p <0.05.
Ethics Study participants provided written informed consent per the Helsinki Declaration.[26] The consent document described the importance of participation and explained the study’s characteristics and possible risks and benefits. All data were kept confidential and participant identity was delinked. The selection of diagnostic tools followed the ethical principles of maximum benefit and nonmaleficence.
Figure 1: Reading window strategies
a. Design for panel 1 of T and NKT cell phenotypesa
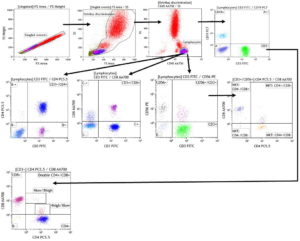
aTo identify double-positive populations, a CD4 vs. CD8 graphic from the CD3+ region was added.
b. Design for activated T lymphocyte panelb
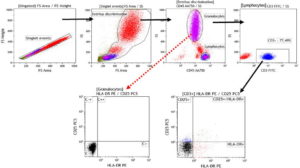
bThe scatter plot marked with a dotted arrow (which comes from the granulocyte region) was used as a control to establish cutoff points for HLA-DR and CD25 tertiary antigens, since these cells do not express these antigens, which were cloned to obtain values for activated subpopulations in the CD3+ region.
NKT: natural killer T
RESULTS
Age did not affect counts and percentages of the cell subpopulations studied; r2 values were distant from unity and differences by age did not meet the specified significance level of p <0.05. For some subpopulations, significant differences were found between men and women.
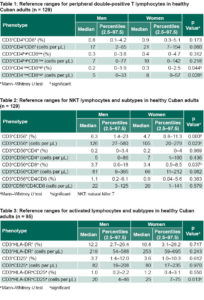
No significant sex differences were found in counts and percentages (of total lymphocytes) of double-positive alpha beta T lymphocytes subpopulations, except for the CD3+CD4lowCD8high phenotype. Median percentages were less than one percent, but the median was one percentage point higher for women than for men. The upper range limit did not exceed 5.1% (Table 1).
Counts and percentages of NKT cells and their subtypes are summarized in Table 2. The CD3+CD56+ phenotype was influenced by sex, with both counts and percentages significantly higher in men (p = 0.003 and p = 0.023, respectively). Percentages of CD3+CD56+CD8+ were also significantly higher in men. For all other subtypes, values were higher in men, but without statistical significance. Wider ranges in counts and percentages were found in this lymphocyte subpopulation than in other subpopulations studied, especially among men.
Counts and percentages of activated lymphocyte subpopulation phenotypes are summarized in Table 3. Modest sex differences were found in subtype medians, with statistical significance for CD3+HLA‒DR+CD25+ (p = 0.013) counts, but not percentage of total lymphocytes (Table 3).
DISCUSSION
Normal blood percentages of rare lymphocytes are influenced by many factors, such as age, sex, viral infections, stress, medications, chronic diseases, lifestyles and even study methodology. Therefore, it is recommended that reference values be established through regional studies and even by countries and that they be updated periodically.[1,2,4,6] To obtain these values and their ranges, immunophenotyping of these subpopulations and their subtypes should be performed using flow cytometry in persons with immune systems unaffected by diseases, treatments or lifestyles. Once determined, they can be used in clinical practice to diagnose and predict the clinical course of immune system diseases and other diseases involving the immune system, such as cancer and infections.[2,4,6]
Values found for the double-positive (DP) alpha beta T population were similar to those published by authors in other countries and showed no differences between men and women.[27–32] However, some studies report an increase in persons aged >60 years.[32] Differences from similar studies are explained by the variety of factors influencing the composition of these populations, which cause great variability in the ranges, underscoring the need to establish country-specific reference values.[28,32]
The population of DP T lymphocytes in the peripheral blood of healthy individuals was described by Nascimbeni in 2004.[27] DP lymphocytes have been associated with antitetanus vaccination, good response to influenza vaccination in older adults, and lymphoproliferation (especially when the monoclonal phenotype is expanded after viral infections such as influenza A or Vaccinia).[28,32] Absence of lymphocytosis is more common in persistent viral infections, either latent or chronic, caused by cytomegalovirus, Epstein–Barr, herpes simplex, varicella zoster, HIV, or hepatitis B or C.[27,28] An increase in DP lymphocytes has been linked to autoimmune diseases (lupus, multiple sclerosis and rheumatoid arthritis), cancer (melanoma and Hodgkin lymphoma) and beta thalassemia (especially after splenectomy). Thus quantification of DP cells is important for prognostic purposes. However, a decrease in or absence of these cells has not been associated with any disease and is considered normal.[6,11,29,30]
DP cell functions are varied due to their double phenotype, with production of cytokine patterns whose balance is related to the most strongly expressed coreceptor, as well as cooperative or cytotoxic functions. In addition, they maintain the ability to recognize antigens presented by both class I and II antigen-presenting molecules.[6,11,28–31] This functional capacity, which demonstrates the lymphoid cell plasticity, allows the immune system to adapt to antigenic challenges, especially long-lasting ones, as in chronic infections and cancer. In the case of HIV infection, their increase has been related to good viral control, even in the acute phase.[32]
DP cells have shown a mainly effector memory phenotype combined with markers of replicative senescence, suggesting that they have undergone continued stimulation over time.[28] This may explain the great variability observed, since they will be more or less abundant depending on the number of exposures to different antigens.
If this DP subpopulation in blood is not considered when performing cell counts, there will be incorrect duplication of their markers, biasing calculation of the CD4/CD8 index. This is a serious problem in followup of HIV patients, since this index requires precision to be useful for classifying patients for antiretroviral therapy, assessing antiviral resistance, monitoring treatment adherence and detecting possible evolution towards AIDS.
NKT populations are characterized mainly by a CD3+, CD56+ phenotype and in humans by absence or differential expression of CD4 or CD8. In 1995, the first articles were published about lymphocyte populations that not only had T cell receptor and NK markers, but also showed a unique type of receptor chain rearrangement and a frequency well above the expected for a specific rearrangement. These cells are called invariant NKT cells.[18–19]
The NKT ranges we found are similar to those reported by Rojas-Pandales in Colombia, who did not observe sex or age effects.[33] The great interindividual variability and wide ranges can be related to the biological characteristics of these lymphocytes, which accumulate in blood as they encounter their cognate antigens. Each person’s NKT levels depend on the number of previous exposures to different agents capable of stimulating this cell population. However, the range for the normal (healthy) population in a given country has lower and upper limits, which allows detection of abnormal findings.[18,23,34,35]
Increased NKTs have been observed in chronic infections, allergies, cancer and autoimmune diseases. However, values can also be normal or decreased in allergies and cancer. Decreased values indicate poor cancer prognosis. NKTs comprise different subpopulations with functional diversity. NKT CD8+ and NKT CD4−CD8− phenotypes are a source of interferon alpha and tumor necrosis factor gamma, which possess obvious antitumor activity and are cytotoxic due to perforin secretion and binding of Fas/Fas-L (molecules that mediate apoptosis). NKT cells recognize nonprotein antigens coupled to CD1 molecules on cell surfaces, detecting malignant cells by recognizing antigens missed by their conventional T cell counterparts. This shows that NKTs complement cancer defense and immunosurveillance differently than classical T and NK cells.[24,36–39]
Among lymphocyte activation markers, HLA-DR and CD25 showed great variability among individuals, with an especially wide range in HLA-DR. This may be related to the presence of effector memory populations of T lymphocytes in blood, which also express these molecules, and to the fact that the size of this subpopulation is determined, among other factors, by the number of previous personal exposures to different immunogens.[40]
These molecules are classified as T lymphocyte late activation antigens and are synthesized after the cellular activation process has begun;[40] in flow cytometry interpretation, they are considered tertiary. Since expression cannot be predicted until cellular activation has occurred, they could influence range variability. Some authors report that age and sex do not greatly influence these expression levels, but do affect the process triggering such activation.[41] Considering that regulatory T cells characteristically express the CD25 marker, measuring lymphocyte activation by other markers, such as HLA-DR is more reliable.[40–43] One trait that can inform flow cytometry analysis is that regulatory T cells express CD25 more intensely, so that it does not behave like a typical tertiary marker.[44]
Expression of markers that reflect cell function are important to assess the activation state of the T cell compartment in patients with autoimmune diseases, because such markers discriminate between the active and compensated states of the disease.[45] There is evidence that the greater the number of activated T cells, the greater the severity of decompensation, through autoimmune pathogenesis. This has been observed in diseases such as multiple sclerosis, Wegener granulomatosis, Kawasaki disease, systemic lupus erythematosus and autoimmune aplastic anemias.[46,47] HLA-DR is used as a marker to predict therapeutic response in autoimmune diseases such as Kawasaki disease, in which patients with a higher percentage of HLA-DR+ T cells fail to respond to therapy with intravenous immunoglobulins.[45–47]
In cancer, activation is analyzed by cell population. Activation of suppressor T cells indicates worsened clinical evolution and poorer prognosis. Evidence indicates correspondence between blood and tumor microenvironments, so that, if activated suppressor cells in the blood increase, so do tumor-infiltrating lymphocytes. But if the activated cells are cytotoxic, this increase can be beneficial, because they are known to help eliminate tumor cells.[43,44]
In the course of infections such as HIV, chronic immune activation is part of immunopathogenesis and is one of the most damaging phenomena in disease progression, since it increases viral load and decreases CD4+ lymphocytes. Monitoring expression of CD25 and HLA-DR, as markers of the degree of T cell activation in these patients, has been found to be very useful.[47]
It is important to consider activation markers to assess immune system function in many diseases, in some cases as an immunopathogenic factor and in others as pathological or physiological consequence of a previous illness or condition. For example, increased HLA-DR expression has even appeared in patients with chronic spinal cord injuries, possibly contributing to persistence of chronic inflammation or decreased resistance to infection.[44]
The levels of T-lymphocyte populations we observed are similar to those found in healthy controls in published research.[43–47]
CONCLUSIONS
Age does not affect counts and percentages of rare T lymphocyte subpopulations in the blood of healthy Cuban adults. Sex differences found for some phenotypes suggest the need for different reference values for women and men.
References

- Zhang K, Wang F, Zhang M, Cao X, Yang S, Jia S, et al. Reference ranges of lymphocyte subsets balanced for age and gender from a population of healthy adults in Chongqing district of China. Cytometry B Clin Cytom. 2016 Nov;90(6):538–42.
- Melzer S, Zachariae S, Bocsi J, Engel C, Loffler M, Tárnok A. Reference intervals for leukocyte subsets in adults: results from a population-based study using 10-color flow Cytometry. Cytometry B Clin Cytom. 2015 Jul–Aug;88(4):270–81.
- Sorrenti V, Marenda B, Fortinguerra S, Cecchetto C, Quartesan R, Zorzi G, et al. Reference values for a panel of cytokinergic and regulatory lymphocyte subpopulations. Immune Netw. 2016 Dec;16(6):344–57.
- Cóndor JM, Álvarez M, Cano L, Matos E, Leiva C, Paredes JA. Intervalos de referencia de subpoblaciones linfocitarias de sangre periférica en adultos sanos de Lima, Perú. Rev Peru Med Exp Salud Publica. 2013;30(2):235–40. Spanish.
- Craig FE, Foon KA. Flow cytometric immunophenotyping for hematologic neoplasms. Blood. 2008 Apr 15;111(8):3941–67.
- Quandt D, Rothe K, Scholz R, Baerwald CW, Wagner U. Peripheral CD4CD8 double positive T cells with a distinct helper cytokine profile are increased in rheumatoid arthritis. PLoS One. 2014 Mar 25;9(3):e93293.
- Voelkl S, Gary R, Mackensen A. Characterization of the immunoregulatory function of human TCR-αß+ CD4- CD8- double-negative T cells. Eur J Immunol. 2011 Mar;41(3):739–48.
- Besedovsky L, Dimitrov S, Born J, Lange XT. Nocturnal sleep uniformly reduces numbers of different T-cell subsets in the blood of healthy men. Am J Physiol Regul Integr Comp Physiol. 2016 Oct 1;311(4):R637–R42.
- Gómez A, González C, Ávila LM, Casas MC, Padilla S. Coexpresión de CD4 y CD8 en linfocitos de sangre periférica en pacientes positivos para VIH. Asoc Colomb Infectol. 2008 Dec;12(4):267–76. Spanish.
- Yazici S, Bülbül Başkan E, Budak F, Oral B, Adim SB, Ceylan Kalin Z, et al. Flow cytometric analysis of T, B, and NK cells antigens in patients with mycosis fungoides. J Immunol Res [Internet]. 2015 [cited 2017 Jul 8];2015:856340. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/26788525/
- Zahran AM, Saad K, Elsayh KI, Alblihed MA. Characterization of circulating CD4+ CD8+ double positive and CD4− CD8− double negative T‑lymphocyte in children with β‑thalassemia major. Int J Hematol. 2017 Mar;105(3):265–71.
- Van Acker HH, Capsomidis A, Smits EL, Van Tendeloo VF. CD56 in the immune system: more than a marker for cytotoxicity? Front. Immunol. 2017 Jul 24;8:892.
- Lambert C, Genin C. CD3 bright lymphocyte population reveal γδ T cells. Cytometry B Clin Cytom. 2004 Sep;61(1):45–53.
- Paget C, Chow MT, Gherardin NA, Beavis PA, Uldrich AP, Duret H, et al. CD3bright signals on γδ T cells identify IL-17A-producing Vγ6Vδ1+ T cells. Immunol Cell Biol. 2015 Feb; 93(2):198–212.
- Maecker H, Trotter J. Selecting reagents for multicolor flow cytometry. Application note. BD Biosci [Internet]. 2012 Jan [cited 2017 Jul 8]. Available from: http://www.unav.edu/documents/4576308/a258d356-b38c-4d2d-b322-ef0c6b0308b4
- Kriegel MA, Adam-Klages S, Gabler C, Blank N, Schiller M, Scheidig C, et al. Anti-HLA-DR-triggered monocytes mediate in vitro T cell anergy. Int Immunol. 2008 Apr;20(4):601–13.
- Perez-Andres M, Paiva B, Nieto WG, Caraux A, Schmitz A, Almeida J, et al. Human peripheral blood B-cell compartments: a crossroad in B-cell traffic. Cytometry B Clin Cytom. 2010;78 Suppl 1:47–60.
- Erazo-Borrás LV, Álvarez-Álvarez JA, Trujillo Vargas CM. Linfocitos NKT invariantes: ontogenia, fenotipo y función. Inmunología. 2014 Apr–Jun;33(2):51–9. Spanish.
- Waldowska M, Bojarska-Junak A, Roliński J. A brief review of clinical trials involving manipulation of invariant NKT cells as a promising approach in future cancer therapies. Cent Eur J Immunol. 2017;42(2):181–95.
- Apoil PA, Puissant-Lubrano B, Congy-Jolivet N, Peres M, Tkaczuk J, Roubinet F, et al. Reference values for T, B and NK human lymphocyte subpopulations in adults. Data Brief. 2017 Apr 21;12:400–4.
- Vély F, Barlogis V, Vallentin B, Neven B, Piperoglou C, Ebbo M, et al. Evidence of innate lymphoid cell redundancy in humans. Nat Immunol. 2016 Nov;17(11):1291–9.
- Zook EC, Kee BL. Development of innate lymphoid cells. Nat Immunol. 2016 Ju 21;17(7):775–82.
- Michel JJ, Griffin P, Vallejo AN. Functionally diverse NK-Like T cells are effectors and predictors of successful aging. Front Immunol. 2016 Nov 24;7:530.
- Berzins SP, Smyth MJ, Baxter AG. Presumed guilty: natural killer T cell defects and human disease. Nat Rev Immunol. 2011 Feb;11(2):131–42.
- Tirado-Rodríguez P, editor. Guía clínica para el abordaje de trastornos relacionados con el consumo de alcohol. Andalucía: Consejería de Igualdad y Bienestar Social de Andalucía; 2007. 226 p. Spanish.
- World Medical Association. Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA [Internet]. 2013 Nov 27 [cited 2018 Jun 13];310(20):2191–4.Available from: https://jamanetwork.com/journals/jama/fullarticle/10.1001/jama.2013.281053
- Ghia P, Prato G, Stella S, Scielzo C, Geuna M, Caligaris-Cappio F. Age-dependent accumulation of monoclonal CD4+CD8+double positive T lymphocytes in the peripheral blood of the elderly. Brit J Haematol. 2007 Dec;139(5):780–90.
- Nascimbeni M, Shin EC, Chiriboga L, Kleiner DE, Rehermann B. Peripheral CD4+CD8+ T cells are differentiated effector memory cells with antiviral functions. 2004 Jul 15;104(2):478–86.
- Quandt D, Rothe K, Scholz R, Baerwald CW, Wagner U. Peripheral CD4CD8 double positive T cells with a distinct helper cytokine profile are increased in rheumatoid arthritis. PLoS One. 2014 Mar 25;9(3):e93293.
- Waschbisch A, Sammet L, Schröder S, Lee DH, Barrantes-Freer A, Stadelmann C, et al. Analysis of CD4+ CD8+ double-positive T cells in blood, cerebrospinal fluid and multiple sclerosis lesions. Clin Exp Immunol. 2014 Aug;177(2):404–11.
- Frahm MA, Picking RA, Kuruc JD, McGee KS, Gay CL, Eron JJ, et al. CD4+CD8+ T-cells represent a significant portion of the anti-HIV T-cell response to acute HIV infection. J Immunol. 2012 May 1;188(9):4289–96.
- Herndler-Brandstetter D, Schwanninger A, Grubeck-Loebenstein B. CD4+ CD8+ T cells in young and elderly humans. 2007 Mar;120(3):292–4.
- Rojas-Pandales F, Bolaños N, Mercado M, González JM, Cuéllar A, Cifuentes-Rojas C. Valores de referencia de células asesinas naturales (NK y NKT) en donantes de sangre de Bogotá. Acta Med Colomb. 2007 Jul–Sep;32(3):124–8. Spanish.
- Bisset LR, Lung TL, Kaelin M, Luwing E, Dubs RW. Reference values for peripheral blood lymphocyte phenotypes applicable to the healthy adult population in Switzerland. Eur J Haematol. 2004 Mar;72(3):203–12.
- Apoil PA, Puissant-Lubrano B, Congy-Jolivet N, Peres M, Tkaczuk J, Roubinet F, et al. Reference values for T, Band NK human lymphocyte subpopulations in adults. Data Brief. 2017 Apr 21;12:400–4.
- Ling L, Lin Y, Zheng W, Hong S, Tang X, Zhao P, et al. Circulating and tumor-infiltrating mucosal associated invariant T (MAIT) cells in colorectal cancer patients. Sci Rep. 2016 Feb 3;6:e20358. DOI: 10.1038/srep20358.
- Fernández CS, Kelleher AD, Finlayson R, Godfrey DI, Kent SJ. NKT cell depletion in humans during early HIV infection. Immunol Cell Biol. 2014 Aug;92(7):578–90.
- Gebremeskel S, Slauenwhite D, Johnston B. Reconstitution models to evaluate natural killer T cell function in tumor control. Immunol Cell Biol. 2016 Jan;94(1):90–100.
- Taniguchi M, Harada M, Dashtsoodol N, Kojo S. Discovery of NKT cells and development of NKT cell-targeted anti-tumor immunotherapy. Proc Jpn Acad Ser B Phys Biol Sci. 2015;91(7):292–304.
- Starska K, Głowacka E, Kulig A, Lewy-Trenda I, Bryś M, Lewkowicz P. The role of tumor cells in the modification of T lymphocytes activity — the expression of the early CD69+, CD71+ and the late CD25+, CD26+, HLA/DR+ activation markers on T CD4+ and CD8+ cells in squamous cell laryngeal carcinoma. Part I. Folia Histochem Cytobiol. 2011;49(4):579–92.
- Lúdvíksson BR, Sneller MC, Chua KS, Talar-Williams C, Langford CA, Ehrhardt RO, et al. Active Wegener’s granulomatosis is associated with HLA-DR1 CD41 T cells exhibiting an unbalanced Th1-Type T cell cytokine pattern: reversal with IL-10. J Immunol. 1998 Apr 1;160(7):3602–9.
- Geraldes L, Morgado J, Almeida A, Todo-Bom A, Santos P, Paiva A, et al. Expression patterns of HLA-DR+ or HLA-DR– on CD4+/CD25++/CD127low regulatory T cells in patients with allergy. J Investig Allergol Clin Immunol. 2010;20(3):201–9.
- Starska K, Głowacka E, Kulig A, Lewy-Trenda I, Bryś M, Lewkowicz P. Prognostic value of the immunological phenomena and relationship with clinicopathological characteristics of the tumor— the expression of the early CD69+, CD71+ and the late CD25+, CD26+, HLA/DR+ activation markers on T CD4+ and CD8+ cells in squamous cell laryngeal carcinoma. Part II. Folia Histochem Cytobiol. 2011;49(4):593–603.
- Monahan R, Stein A, Gibbs K, Bank M, Bloom O. Circulating T cell subsets are altered in individuals with chronic spinal cord injury. Immunol Res. 2015 Dec;63(1–3):3–10.
- Arneth B. Activated CD4+ and CD8+ T cell proportions in multiple sclerosis patients. 2016 Dec;39(6):2040–4.
- Wakiguchi H, Hasegawa S, Suzuki Y, Kudo K, Ichiyama T. Relationship between T-cell HLA -DR expression and intravenous immunoglobulin treatment response in Kawasaki disease. Pediatr Res. 2015 Apr;77(4):536–40.
- Jaramillo-Ruiz LD, Muñoz-Fernández MA, Correa-Rocha R. Estudio preliminar sobre las alteraciones fenotípicas de las células Treg causadas por la infección VIH en pacientes adultos infectados. Rev Complut Cienc Vet. 2011;5(2):49–64. Spanish.
THE AUTHORS
Carlos A. Villegas-Valverde (Corresponding author: carlosvillega@infomed.sld.cu), physician with dual specialties in family medicine and immunology and master’s degrees in infectious diseases and medical education. Associate researcher, National Oncology and Radiobiology Institute, Havana, Cuba; and associate professor, Medical University of Havana, Cuba.
Elena Kokuina, physician specializing in immunology, Hermanos Ameijeiras Clinical–Surgical Teaching Hospital, Havana, Cuba.
Martha C. Breff-Fonseca, physician specializing in clinical laboratory medicine, Hermanos Ameijeiras Clinical–Surgical Teaching Hospital, Havana, Cuba.
Submitted: December 18, 2017 Approved: October 06, 2018 Disclosures: None






