As a result of accelerated training of scientists and engineers beginning in the 1960s and substantial state investment since the 1980s calculated at about USD$1 billion,[1] Cuban biotechnology has become a major player in the Cuban economy and successfully established its place in the global market. According to a World Bank report, Cuba has the capacity to meet about 80% of its domestic demand for prescription drugs and had achieved annual biotech exports of around USD$100 million in the 1990s.[2] A recent Ernst & Young report puts exports of biotech products at USD$300 million in 2005.[3]
Behind the commercial success of Cuba’s biotech industry are several innovative vaccines, including the recombinant hepatitis B vaccine, which received pre-qualification from the World Health Organization (WHO) in 2001 for international use and is now sold in more than 30 countries worldwide. Cuba also developed the world’s first effective vaccine against meningitis B, VAMENGOC- BC®, which is now exported primarily to Latin America, including Brazil, Argentina, Colombia, Venezuela, and Uruguay. Based on this successful experience, in 1999, the British company Glaxo-Smith-Kline entered into a licensing agreement with Cuba’s Finlay Institute to develop a meningococcal B vaccine using Cuban technology, to be distributed in Europe and North America.
International acceptance of Cuban biotech is growing, with products now registered in more than 55 countries, principally in the Global South. A deep project pipeline, including innovative therapeutic cancer vaccines – currently in clinical trials in Cuba, Canada, and elsewhere – mean the country’s market share is likely to keep growing. By 2004, Cuba had registered some 100 patents and applied for another 500 patents throughout the world.[4] Cuba’s safety and research standards are reported to “[be] equal or even exceed those of the US Federal Drug Administration and the European Union.”[5]
The success of Cuba’s program has also brought unwelcome controversy however. In May 2002, Under Secretary of State for Arms Control and International Security, John Bolton, alleged that Cuba had a biological weapons program – an allegation that has never been proven. First-hand reports cast doubt on the veracity of the claim and a delegation of experts sponsored by the Center for Defense Information in 2003 concluded that it is unlikely that Cuba has a bio-weapons program.[6] In a 2005 State Department report, the US government admitted that it has no firm evidence that Cuba has such a program.[7] Finally, Cuba has complied with the reporting requirements of the Secretariat of the Biological Weapons Convention since 1992. In November 2001, the Secretariat found that Cuba had no biological weapons research programs.[8]
The Cuban Approach
Two factors differentiating the Cuban biotech industry are the high level of integration and cooperation among scientific institutions, and their “closed loop” approach, which emphasizes translational research and coordinates the entire process among institutions – from research to marketing – of any given biotech product. (See Viewpoint: Connecting Science to Population Health: The “Closed Loop” Approach). As noted in a 2004 study in Nature Biotechnology, “knowledge sharing among and within the research institutions is an important feature” that is highly encouraged through the system.[4] For example, Cuba’s Haemophilus influenzae type b vaccine (Hib), the world’s first synthetic antigen vaccine, was the result of the collaboration by five institutions: the University of Havana, Center for Genetic Engineering and Biotechnology (CIGB), the Finlay Institute, the National Center for Bioproducts, and the Pedro Kourí Institute for Tropical Diseases (IPK). This vaccine will be submitted to the WHO for prequalification for international use.
Cuban research also prioritizes developing affordable vaccines for diseases affecting poor populations, such as typhoid fever and cholera: a fundamentally needsdriven, rather than market-driven approach. This can be contrasted with transnational pharmaceutical companies, which have come under increasing criticism for placing market interests before global health solutions, resulting in investment of 90% of R&D dollars worldwide in developing treatments for diseases affecting the 10% of the world’s population that can afford the results. Cuba also produces generic drugs, including HIV/AIDS antiretrovirals, selling them to developing countries at a fraction of the price sold by transnationals.[9]
Another hallmark of Cuba’s biotechnology program is its joint venture projects with other countries. In addition to collaborative ventures with Canada and Great Britain, Cuba presently licenses its biotechnology and has joint projects in a growing number of developing countries around the world, including Algeria, Brazil, Canada, China, India, Malaysia, Mexico, South Africa, Tunisia, and Venezuela. In addition, China has become a major participant in Cuban biotech projects; three joint ventures have been formed in China using Cuban technology.
The Scientific Pole
Although the Cuban government established the National Center for Scientific Research in 1965, biotechnology did not emerge as an important branch of the Cuban economy until 1986 with the founding of the Center for Genetic Engineering and Biotechnology. Today, the main biotech institutions belong to what is known as the Scientific Pole which boasts ten major centers and more than 50 related research and production institutions, employing more than 1,500 researchers. The following is a brief description of these 10 core institutions.
Center for Genetic Engineering & Biotechnology (CIGB)
Sometimes referred to as Cuban biotech’s flagship research center, CIGB is a large research-production complex, with some 550 scientists and engineers on staff, devoted primarily to the development of vaccines, pharmaceuticals, and plant molecular biology. Its vaccine division is developing new formulations using genetic engineering techniques. CIGB, together with four other institutions, developed the world’s first vaccine using a synthetic antigen against Haemophilus influenzae type b. It also markets a pentavalent vaccine, developed with the Finlay Institute, against diphtheria, tetanus, pertussis, hepatitis B, and Haemophilus influenzae type b. Among its leading exports are a recombinant hepatitis B vaccine (HEBERBIOVAC-HB) and the Gavac™ vaccine against Boophilus microplus cattle ticks. Other important recombinant products are Epidermal Growth Factor (EGF), recombinant Streptokinase, and γ Interferon. In 1991, CIGB established Heber Biotec, S.A., a subsidiary with exclusive rights to market CIGB’s products worldwide, and which has joint ventures with companies in India and China.
The Scientific Pole: Publications
In addition to research and development, Scientific Pole researchers are increasingly publishing results in international journals of impact and in their own centers’ publications; most are peer reviewed and include abstracts in English.
CENSA
Revista de Protección Vegetal (ISSN 1010-2752)
Revista de Salud Animal (ISSN 0253-570X)
CIGB
Avances en Biotecnología Moderna (ISSN 1027-2860)
Biotecnología Aplicada (ISSN 1027-2852)
CNIC
Ciencias Biológicas (ISSN 0258-6002)
Ciencias Químicas (ISSN 0254-0525)
Finlay Institute
VacciMonitor (ISSN 1025-0298)
IPK
Revista Cubana de Medicina Tropical (ISSN 0375-0760)
Boletín Epidemiologico Semanal (ISSN 1028-5083)

The Finlay Institute
Founded as an institution in 1991 on the basis of several years’ work by its leading scientific team, the Finlay Institute is central to Cuba’s vaccine research and production capability: more than a third of the vaccines currently being researched in Cuba are being developed at Finlay. Its most successful and best known product is the world’s first effective meningitis B vaccine, VAMENGOC- BC®, used as a B and C combination vaccine. Finlay’s product portfolio also includes vaccines against leptospirosis, typhoid fever (Vi polysaccharide), and tetanus. Their combination vaccines include the bivalent vaccine against diphtheria and tetanus for children and adults and a trivalent vaccine against diphtheria, tetanus, and pertussis (whooping cough). Finlay has approximately 900 employees, over 500 of whom are scientists and engineers. (See Interview: Concepción Campa-Huergo.)
Center for Molecular Immunology (CIM)
One of Cuba’s newest research centers founded in 1994, CIM’s research and development is focused primarily on the development of monoclonal antibodies for diagnosis and treatment of cancer. Its innovative therapeutic cancer vaccines are in various phases of clinical trials in Cuba, Canada, and elsewhere. Through its marketing company, CIMAB, S.A., it has formed joint ventures with Canadian (YM Biosciences Inc.), Chinese (Center of International Science), and Indian (Biocon India) companies. In 2003, the US company CancerVax obtained a license from the US Treasury Department enabling it to conduct clinical trials for one of CIM’s cancer treatments in the US.
The National Center for Bioproducts (CNB)
Consisting of six different plants with modern facilities, CNB, which began operations in 1992, primarily produces bioreagents, including over 50 types of culture media for use in microbiology, laboratory, and industrial biotechnology. It also produces the hepatitis B recombinant vaccine, developed in collaboration with CIGB, as well as the tetravalent and pentavalent vaccines, for domestic use and export. It has the production capacity to produce 14,000 doses per hour. CNB also has several specialized laboratories where it conducts research related to allergy vaccines and produces an erythropoiesis-stimulator (TROFIN®), a natural product used in the treatment of anemia.
Pedro Kourí Tropical Medicine Institute (IPK)
Founded in 1937 as part of the University of Havana Medical School, IPK moved to a new modern facility in 1993 where its staff of over 380 scientists and technicians conduct research on tropical diseases, including parasitology. In recent years, IPK has become more involved in medical microbiology, infectious diseases, epidemiology, and evaluating vaccine clinical trials. It is also a teaching hospital with 200 beds providing clinical services to HIV+ patients. IPK has also developed an international reputation for its dengue fever research.
The Scientific Pole: World Intellectual Property Organization Gold
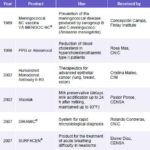
Source: www.ocpi.cu/premios02.htm
Center for Immunoassays (CIE)
One of the smaller centers, CIE has a national network of laboratories for diagnosing infectious diseases. It produces the ultra-micro-ELISA diagnostic kits and equipment used for testing for HIV, hepatitis B and C, dengue, Chagas, and other diseases. Its marketing branch, Tecnosuma SA, sells its products internationally.
National Center for Neurosciences
Founded in 1990, this center develops and produces medical equipment and testing devices for diagnosis and treatment of neurological disorders. It has developed an innovative diagnostic tool for detecting early auditory damage in children younger than age one.
National Center for Scientific Research (CNIC)
One of Cuba’s first biotechnology research centers, CNIC was founded in 1965 and has conducted research in a variety of fields, including the development of pharmaceutical products. It is perhaps best known for PPG, a popular anti-cholesterol drug produced from a sugar cane byproduct. In addition, it has done important work on clinical uses of ozone as well as the invention of equipment for rapid microbiological diagnosis (DIRAMIC®).
National Center for the Production of Laboratory Animals (CENPALAB)
Established in 1982, CENPALAB moved to a new facility in 1992. As its name suggests, it raises and supplies animals for laboratory research and preclinical and quality control testing by the other research and production institutes. CENPALAB worked with CIGB to develop the vaccine against Boophilus microplus cattle ticks.
National Center for Agricultural & Animal Health (CENSA)
Founded in 1969, and installed in a new facility in 1980, CENSA is involved in research focused on plant and animal health and produces vaccines, medications, and diagnostic techniques for veterinarian agricultural and human use. Its research regarding natural pesticides and protection against plagues has been important to the growth of Cuba’s potato, sugar cane, tomato, banana, and coffee crops. It also focuses on improvement of milk production. With respect to human health, CENSA produces a pulmonary surfactant (SURFACEN®) that has prevented the death of premature babies.
References

- Lopez E, Acevedo BE, Silva R, Tormo B, Montero R, Herrera L. Development of Cuban biotechnology. Jour of Comm Biotech. 2002;9(2):147-152.
- Kaplan W, Laing R. Local Production of Pharmaceuticals: Industry Policy and Access to Medicines. Health, Nutrition and Population Discussion Paper. The World Bank. 2005 Jan;16.
- Ernst & Young. Doing Business with Cuba: Update. Cuba Absolutely. 2007;Vol 1:55.
- Thorsteinsdottir H, Sáenz WT, Quach U, Singer PA, Daar AS. Cuba – innovation through synergy. Nature Biotechnology. Dec 2004;22 Suppl:19-24.
- Fineman M. Little-known biotech industry vital to Cuba’s economic future. Miami Herald. 1998 Aug 14.
- Baker G, editor. Cuban Biotechnology: A First Hand Report. Center for Defense Information. 2003.
- Bureau of Verification and Compliance; US State Department. Adherence to and Compliance with Arms Control, Nonproliferation, and Disarmament Agreements and Commitments. Washington DC; 2005 Aug 30.
- BWC/C/CONF. V/3/Add. 7, Nov 28, 2001.
- Department of Foreign Affairs and International Trade, Canada; The Market Brief: The Biotechnology Market in Cuba. Market Resource Centre. Ottawa; 2003 March.






