ABSTRACT
Pleural effusion is a common condition in critically ill patients (both clinical and surgical). Its diagnosis and classification are important for followup of patients with cardiorespiratory difficulty. Lung ultrasound is used for this purpose, but no reports have been published on its use in Cuba with critically ill patients in intensive care units. We performed lung ultrasound on 144 such patients with cardiorespiratory illnesses, average age 54 years, predominantly men (66%; 95/144), with average APACHE II score 13.6, and 22.1% mortality risk. Patients were divided into two groups: clinical (bronchopneumonia and cardiac insufficiency) and surgical (postoperative liver and kidney transplant or vascular and cardiovascular surgery) to diagnose and classify pleural effusion according to locus (right, left and bilateral) and structural pattern (I, II A, II B, III and IV). Pleural effusions were diagnosed in 81.2% (117/144) of patients (clinical 44.4%, 52/117; surgical 55.6%, 65/117). Bilateral location was the most common (68.4%, 80/117), followed by right (23.9%, 28/117) and then left (7.7%, 9/117). Structural pattern I (anechoic appearance) was observed in 61.5% of cases (72/117); 21.4% (25/117) were II A, 12.8% (15/117) II B, 3.4% (4/117) III, and 0.9% (1/117) were IV. We found no association between pleural effusion localization and ultrasound structural pattern in clinical patients (Fisher exact test 4.2 p = 0.9). In surgical patients, however, complex ultrasound patterns (II A, II B and III) were significantly more common in bilateral forms (Fisher exact test 14.1; p = 0.009). Further studies of this type in Cuba will help provide useful data for prompt treatment and followup of these patients.
KEYWORDS Pleural effusion, critical illness, intensive care unit, lung ultrasound, diagnostic ultrasound, Cuba
INTRODUCTION
Pleural effusion (PE) is a common illness in intensive care units (ICUs). Critically ill patients develop PE as an outcome of a primary disease or as a side effect of treatment and life-support measures.[1] PE diagnosis in critically ill patients traditionally has been based on simple anteroposterior x-rays.[2] Lung ultrasound (LU) has several advantages over this method: it takes less time, is reproducible in real time, and can be repeated as necessary. In critically ill patients, it provides better sensitivity and reliability for PE diagnosis.[3]
LU can also be performed at bedside, important since transporting ICU patients to a radiology department poses high risk of complications and adverse events. Acute hemodynamic and respiratory complications may occur in 40%–50% of patients with severe respiratory difficulty during transfer.[3] In trauma patients, a baseline CT scan performed within 12 hours of admission to ICU informs a change in clinical management in less than 30% of cases but exposes patients to transfer-related risks. The potentially hazardous effects of exposure to radiation or a contrast medium associated with these procedures should also be considered.[3]
Since the 1967 publication of Joyner’s study describing the reflected ultrasound technique to diagnose PE, major advances have been made in such imaging technology and professional training for its use.[4] These have led to clinical practice guidelines on LU use for PE diagnosis and treatment.[5]
IMPORTANCE This article presents the results of Cuba’s first report on use of lung ultrasound in diagnosis and classification of pleural effusion in critically ill patients in intensive care units.
International evidence-based recommendations indicate that point-of-care LU is more accurate in PE detection than supine radiology and as accurate as CT.[6] Chest x-rays can detect PE in upright patients only when effusion volume is >200 mL; this method’s sensitivity decreases in the supine position, while ultrasound may detect an effusion as small as 20 mL.[7,8] Moreover, ultrasound can detect the morphology and locus of pleural effusion and other associated conditions (such as atelectasis and diaphragmatic disorders). Baseline LU is recommended before thoracentesis, in order to reduce complications.[9]
Cuba’s first published reference to LU use was authored by a pediatrician in 1987.[10] Several Cuban ICUs have used LU, including the ICU in Cuba’s Medical-Surgical Research Center (CIMEQ), which began applying the procedure seven years ago. However, no studies have been published in Cuba on the application of LU to diagnosis and classification of PE in ICU patients.
INTERVENTION
Objective Diagnose and classify PE in critically ill patients in a Cuban ICU using LU.
Justification PE diagnosis and classification is quite important to determine appropriate procedures to manage critically ill patients in ICUs. Use of LU serves this goal, with the advantage that it can be performed at bedside, is more portable than CT and MRI, and has the sensitivity features described previously.[3,7,8]
Participants Study participants were patients (n = 144) hospitalized in CIMEQ’s multipurpose ICU in Havana between January 2012 and September 2017, aged >18 years (average 54, SD 16), predominantly men (66%, 95/144). All patients were classified by their Acute Physiology and Chronic Health Evaluation (APACHE II) score,[11] which enables evaluation and confirmation of severity and provides an estimate of an individual’s mortality risk; in some cases it may be a criterion for ICU admission. Average APACH II score was 13.6 (SD 7.6) and corresponding mortality risk was 22.1% (SD 20.3). We classified patients in two groups—clinical (69/144) and surgical (75/144)—according to diagnosis upon ICU admission. Clinical patients presented with illnesses requiring cardiorespiratory monitoring (bronchopneumonia and cardiac insufficiency); surgical patients were in post-operative phase of complex procedures (liver and kidney transplants, vascular and cardiovascular surgeries) who did not present malignancies.
PE variables
- Presence or absence
- Location (right, left, or bilateral)
- Appearance (per Tu classification)[12]
- I: Anechoic: no echogenic density within the effusion;
- II A: Complex nonseptated and relative hyperechoic: predominant hyperechoic spots visible in the effusion, and echogenic shape that does not change with respiration
- II B: Complex nonseptated and relatively nonhyperechoic: some visible bright spots as echogenic density within the effusion and echogenic shape that changes with respiration
- III: Complex septated: prominent fibrinous septation visible within the effusion;
- IV: Homogeneously echogenic: density of echogenic spots evenly distributed within the effusion
Lung ultrasound LU assessments were conducted with ProSound Alpha 5 SV equipment (Aloka, Japan), using a flat or concave 3−5 MHz transducer and a 5−7 MHz flat transducer when needed.
LU is performed with the ICU patient prone. Each hemithorax is divided into four areas and each of these into two zones—anterior and lateral—separated by the anterior axial line. Each zone, in turn, is divided into superior and inferior according to a horizontal line between the middle and lower thirds of the sternum. Diagnosis is performed through the application of transducers directly on the chest. PE is seen as an echo-free space (dark anechoic image) between the parietal and visceral pleurae, with other specific imaging signs.
The ultrasound image of a pleural effusion shows loss of pleural movement. When etiology is infectious, hyperechoic structures are visible in the effusion’s interior or adhered to the pleura; presence of septation indicates chronic conditions. An effusion’s extent can be estimated in several ways, but qualitative characteristics are always more useful for determining prognosis.[13,14]
Data collection and analysis We set up an Excel database and performed data analysis with SPSS version 20. Absolute and relative frequencies were used. We used the Fisher exact test for analysis of associations involving patient type, PE locus, and ultrasound pattern (significance threshold p = 0.05).
Ethics Patients or their relatives (in the case of unconscious or sedated patients) received explanation of the study’s objectives, risks and benefits, and written informed consent was obtained. The study was approved by CIMEQ’s ethics committee.
RESULTS
PE was diagnosed in 81.2% of patients (117/144). Approximately 55.6% (65/117) of PE cases occurred in surgical patients and 44.4% (52/117) in clinical patients. Bilateral effusions were most common at 68.4% (80/117) followed by effusions on the right (23.9%, 28/117) and left side (7.7%, 9/117) (Table 1).
PE ultrasound patterns showed decreasing frequency from simplest to most complex. Pattern I was observed in 61.5% of cases (72/117), pattern II A in 21.4% (25/117) and pattern II B in 12.8% (15/117). The septated pattern III was observed in 3.4% (4/117) and pattern IV in a single patient for 0.9% (1/177) (Table 1).
In clinical patients, no association was found between PE location and ultrasound pattern (Fisher exact test 4.28, p = 0.96). In surgical patients, however, we found an association (Fisher exact test of 14.16, p = 0.011) between bilateral localization and more complex ultrasound patterns (II A, II B and III).
LESSONS LEARNED
Compared to other studies, ours found higher frequency of PE diagnosed via LU. One study in a US ICU reported 62% (62/100)[15] and another in an Egyptian multipurpose ICU reported 60% (78/130) in patients with pleuropulmonary disorders.[16] Results of a German study carried out in a surgical ICU were similar to ours, as PE was diagnosed in 73% of cases (35/48).[17] Our study was conducted in a multipurpose ICU where patients with complex surgeries receive postoperative care. To maintain proper hemodynamic status during surgery, large volumes of liquid are administered intravenously. This necessary overhydration may increase circulating volume and cause increased pleural capillary pressure with a decrease in reabsorption, thereby producing PE.[18] Most PE patients in our study were in the surgical group. From these observations, we infer that PE is a common disorder in ICUs, especially in surgical patients.
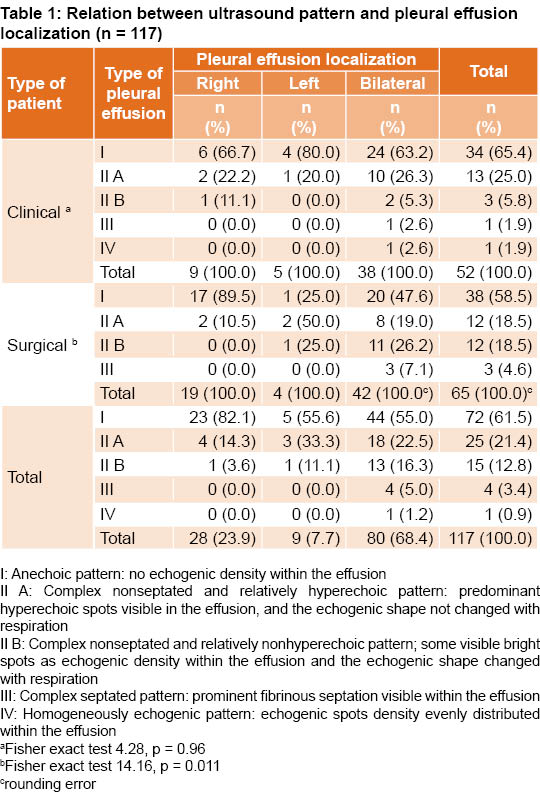
Unlike results reported by other authors, where the right hemithorax was the most common locus of PE, our study found the bilateral most prevalent. In a Cuban internal medicine clinic, Pérez Bada found that 63.6% (35/55) of cases presented on the right side, 23.6% (13/55) on the left, and 12.7% (7/55) bilaterally.[19] In pediatric clinics in Cuba’s Sancti Spíritus Province, right pleural effusion was the most common with 66.7% (10/15).[20] Although these were not ICU patients, we mention these findings here as the only comparisons available for Cuba. A study in Nigeria found PE in the right hemithorax in 50.2% (107/213) of cases, in the left in 42.7% (91/213), and bilaterally in 5.6% (12/213).[21] In a hepatic hydrothorax study in Madrid, the right side also predominated (85.4%).[22] In Tu’s study, right localization was most common with 39.4% (37/94), followed by left with 30.8% (29/94), then bilateral with 29.8% (28/94).[12]
PE location varies and depends on the site of the original disease causing PE (viral or bacterial pneumonia, pulmonary thromboembolism, tumor), and may be localized in either the left or right hemithorax or both.[1,2] The main cause of bilateral PE is congestive heart failure. Other causes are liver diseases, severe kidney failure, hypoalbuminemia and fluid overload.[23,24] All these conditions occur more frequently in ICUs, mainly those receiving patients directly out of surgery. The predominance of bilateral PE in our study can be explained by the high proportion of surgical patients. Bilateral PE is the most common in critically ill patients with diseases requiring cardiorespiratory monitoring (bronchopneumonia and cardiac insufficiency) or following major surgery (liver or kidney transplants, vascular or cardiovascular surgeries).
There are several ultrasound classifications of pleural effusion. Some use two categories (simple or complex)[25] and others, including our study, define pleural effusion’s imaging characteristics in greater detail.[12] We found anechoic patterns to be more common than hyperechoic patterns, with a distribution from lesser to greater morphological complexity. Tu’s work—the main reference for our study—presented these results: pattern I: 39.8% (47/118), II A: 30.5% (36/118), II B: 1.7% (2/118), III: 9.3% (11/118), and IV: 1.7% (2/118).[12] Our study was similar in that it showed predominantly anechoic patterns (I and II A) and differed in the frequency of cases with septated patterns (II B, III, and IV).
Other studies have also observed a predominantly anechoic pattern. Chen,[26] for example, reported anechoic in 44.9% (57/127) and a complex nonseptated pattern in 55.1% (70/127), with no complex septated or homogeneously echogenic patterns. Similar to our study, Lomas reported that 60% (54/90) presented anechoic appearance; the remaining patients (40%, 36/90) presented with diffuse internal echoes with internally septated primary loculation, and others showed no distinction between solid and liquid ultrasound features in the pleural space.[27]
Echogenic PEs ranged from lesser to greater structural complexity with a smaller proportion of patients presenting the most complex (II B, III and IV) patterns. With respect to the association between PE locus and classification according to ultrasound pattern, our results suggest that the bilateral pleural effusions of surgical patients present more complex ultrasound patterns (not seen in clinical patients).
The literature review did not reveal a relationship between patient type and localization or morphology of their PEs, with the exception of Tu’s study.[12] Differences between Tu’s findings and our study may be partly due to Tu’s subjects consisting of febrile ICU patients. In Tu, cases complicated with empyema presented the following distribution: 66.7% (10/15) ultrasound patterns II B and IV, none with patterns I and II A, and 33.3% (5/15) pattern III. PE localized on the right hemithorax was the most common with 53.3% (8/15), followed by bilateral with 26.6% (4/15), and lastly on the left with 20% (3/15).[12] Tu’s study shows that in severe diseases of the pleura (empyema), complex structural patterns predominated.
Our study concurs with Tu[12] in finding that lung disease severity affects complex ultrasound patterns. We also observed association in surgical patients between incidence of bilateral PE and more complex structural patterns detected by LU.
Although results from a single ICU and a relatively small sample cannot be generalized, these are the first findings describing diagnosis and classification of PE with LU in a Cuban ICU context and thus serve as a reference point for further research.
This application of LU in Cuba confirms what has been learned elsewhere, that it is feasible in any appropriately equipped ICU. Compared to other imaging techniques, the procedure is simple and inexpensive, and personnel can be easily trained. We recommend study replication in other Cuban hospitals to obtain additional data useful for timely training and patient followup.
References

- Pneumatikos I, Bouros D. Pleural effusions in critically ill patients. Respiration. 2008;76(3):241−8.
- Trotman-Dickenson B. Radiology in the intensive care unit (Part I). J Intensive Care Med. 2003 Jul–Aug;18(4):198‒210.
- Peris A, Tutino L, Zagli G, Batacchi S, Cianchi G, Spina R, et al. The use of point-of-care bedside lung ultrasound significantly reduces the number of radiographs and computed tomography scans in critically ill patients. Anesth Analg. 2010 Sep;111(3):687‒92.
- Joyner CR Jr, Herman RJ, Reid JM. Reflected ultrasound in the detection and localization of pleural effusion. J Am Med Assoc. 1967 May 1;200(5):399‒402.
- Sikora K, Perera P, Mailhot T, Mandavia D. Ultrasound for the detection of pleural effusions and guidance of the thoracentesis procedure. ISRN Emerg Med [Internet]. 2014 Feb [cited 2018 Feb 4]; 2012:10 p. Available from: https://criticalcarethoughtsdotcom.files.wordpress.com/2014/02/sikora-et-al-review.pdf
- Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012 Apr;38(4):577‒91.
- Agricola E, Arbelot C, Blaivas M, Bouhemad B, Copetti R, Dean A, et al. Ultrasound performs better than radiographs. Thorax. 2011;66(9):828‒9.
- Prina E, Torres A, Carvalho CR. Lung ultrasound in the evaluation of pleural effusion. J Bras Pneumol. 2014 Jan–Feb;40(1):1‒5. English, Portuguese.
- Havelock T, Teoh R, Laws D, Gleeson F; BTS Pleural Disease Guideline Group. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. 2010 Aug;65 Suppl 2:ii61‒76.
- Vázquez Ríos B, Razón Behar R. Utilidad de la ultrasonografía en la exploración torácica. Rev Cubana Ped. 1987;19(1):89‒96. Spanish.
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985 Oct;13(10):818‒29.
- Tu CY, Hsu WH, Hsia TC, Chen HJ, Tsai KD, Hung CW, et al. Pleural effusions in febrile medical ICU patients: chest ultrasound study. Chest. 2004 Oct;126(4):1274‒80.
- Cassanelli N, Caroli G, Dolci G, Dell’amore A, Luciano G, Bini A, et al. Accuracy of transthoracic ultrasound for the detection of pleural adhesions. Eur J Cardiothorac Surg [Internet]. 2012 Nov [cited 2018 Feb 8];42(5):813‒8. Available from: https://pdfs.semanticscholar.org/f4b8/921a089b8e6508615fbab0e6481259c09c66.pdf
- Balik M, Plasil P, Waldauf P, Pazout J, Fric M, Otahal M, et al. Ultrasound estimation of volume of pleural fluid in mechanically ventilated patients. Intensive Care Med. 2006 Feb;32(2):318.
- Mattison LE, Coppage L, Alderman DF, Herlong JO, Sahn SA. Pleural effusions in the medical ICU: prevalence, causes, and clinical implications. Chest. 1997 Apr;111(4):1018‒23.
- Elmahalawy II, Doha NM, Ebeid OM, Abdel-Hady MA, Saied O. Role of thoracic ultrasound in diagnosis of pulmonary and pleural diseases in critically ill patients. Egypt J Chest Dis Tuberculosis. 2017 Apr;66(2):261‒6.
- Schleder S, Dornia C, Poschenrieder F, Dendl L, Cojocaru L, Bein T, et al. Bedside diagnosis of pleural effusion with a latest generation hand-carried ultrasound device in intensive care patients. Acta Radiol. 2012 Jun 1;53(5):556‒60.
- Miserocchi G. Mechanisms controlling the volume of pleural fluid and extravascular lung water. Eur Respir Rev. 2009 Dec;18(114):244‒52.
- Pérez Bada E, Rodríguez Antelo R, Marín Torres MA, Ruíz Martínez M, Bermúdez Martín L, Rodríguez Niebla G. Caracterización del derrame pleural en el Servicio de Medicina Interna. Acta Méd del Centro. 2013;7(1):19–26. Spanish.
- Cordeiro Díaz M, Pérez Rodríguez T, Rivero Díaz A. Derrame pleural. Casuística de un año. Rev Cubana Ped. 1987;59(2):257‒63. Spanish.
- Adeoye PO, Johnson WR, Desalu OO, Ofoegbu CP, Fawibe AE, Salami AK, et al. Etiology, clinical characteristics, and management of pleural effusion in Ilorin, Nigeria. Niger Med J. 2017 Mar–Apr;58(2):76‒80.
- Fernández Font JM, Miguel Díez JD, Opio Maestro V. Hidrotórax hepático. An Med Interna. 2005 May;22(5):248‒57. Spanish.
- Ferreiro L, San José E, Antelo JS, Valdés L. Bilateral pleural effusion: a proposed diagnostic decision algorithm. Ann Am Thorac Soc. 2016 Oct;13(10):1865‒7.
- Puchalski JT, Argento AC, Murphy TE, Araujo KLB, Oliva IB, Rubinowitz AN, et al. Etiologies of bilateral pleural effusions. Respir Med. 2013 Feb;107(2):284‒91.
- Yang PC, Luh KT, Chang DB, Wu HD, Yu CJ, Kuo SH. Value of sonography in determining the nature of pleural effusion: analysis of 320 cases. AJR Am J Roentgenol. 1992 Jul;159(1):29‒33.
- Chen HJ, Tu CY, Ling SJ, Chen W, Chiu KL, Hsia TC, et al. Sonographic appearances in transudative pleural effusions: not always an anechoic pattern. Ultrasound Med Biol. 2008 Mar;34(3):362‒9.
- Lomas DJ, Padley SG, Flower CD. The sonographic appearances of pleural fluid. Brit J Radiol. 1993 Jul;66(787):619‒24.
THE AUTHORS
Alain Cueto-Medina (Corresponding author: acuetom@infomed.sld.cu), physician with dual specialties in family medicine and emergency medicine and a master’s degree in urgent care medicine. Associate professor, Intensive Care Unit, Medical-Surgical Research Center (CIMEQ), Havana, Cuba.
Pablo Lino Alonso-Díaz, physician specializing in emergency medicine with a master’s degree in atherosclerosis research. Associate professor, Intensive Care Unit, CIMEQ, Havana, Cuba.
Rodolfo Martínez-Casanova, physician specializing in emergency medicine. Instructor, Intensive Care Unit, CIMEQ, Havana, Cuba.
Franklin Porto-González, physician specializing in emergency medicine, Intensive Care Unit, CIMEQ, Havana, Cuba.
Yalina Quevedo-Benítez, cardiologist, Intensive Care Unit, CIMEQ, Havana, Cuba.
Anselmo A. Abdo-Cuza, physician specializing in emergency medicine with a doctorate in medical sciences. Full professor, Intensive Care Unit, CIMEQ, Havana, Cuba.
Submitted: October 03, 2018 Approved: January 15, 2019 Disclosures: None




