INTRODUCTION
Tuberculosis (TB), a disease caused by Mycobacterium tuberculosis, is a serious public health problem worldwide, the second leading infectious cause of death after HIV.[1,2] The discovery of antituberculosis drugs in the last century led to a substantial reduction in TB morbidity and mortality, but emergence and spread of strains resistant to first- and second-line antituberculosis drugs are currently important obstacles to TB control worldwide.[1,2]
M. tuberculosis drug resistance happens naturally as a result of spontaneous genetic mutations occurring in the bacterial chromosome. Resistant mutations are rare in wild M. tuberculosis populations, but inadequate use of drugs for treatment has led to an increase in resistant strains.[3]
Current knowledge of M. tuberculosis drug resistance is the result of implementation of the Global Project on Anti-Tuberculosis Drug Resistance Surveillance, launched in 1994 to collect and assess data on antituberculosis drug resistance systematically and continuously worldwide. Surveillance can be conducted by systematically applying sensitivity tests to all patients diagnosed with TB or by carrying out periodic surveys using randomly selected samples of diagnosed patients.[4]
According to the WHO 2015 Global Tuberculosis Report, there were an estimated 9.6 million new TB cases worldwide in 2014, 12% of which were HIV-positive.[2] Resistance surveillance data indicate that multidrug-resistant (MDR, resistant to at least isoniazid and rifampicin) TB occurred in 3.3% of new cases and in 20% of patients with a history of antituberculosis drug treatment. As in previous years, the highest numbers of MDR-TB were recorded in Eastern Europe and Central Asia. At the global level, 12% of 2.7 million bacteriologically-confirmed new cases and 58% of 0.7 million previously treated cases identified in 2014 underwent sensitivity tests, an increase over 2013, when 8.9% of new and 17% of previously treated cases had sensitivity tests.[1,2] Extensive drug resistance (XDR) was identified in 9.7% of MDR cases, with resistance also to a fluoroquinolone (FQ) and one of the second-line injectable drugs. By 2014, at least one case of XDR-TB had been reported in 105 countries.[2]
Detection and treatment of MDR-TB remains top priority for TB control in the Americas. In 2011, 29 of the Region’s 35 countries reported data on performance of sensitivity assays. Coverage was poor, with assays done in only 11% of new and 19% of previously treated cases.[5] In 2012, there were approximately 7000 cases of MDR-TB in the Americas Region, representing approximately 2% of new pulmonary cases and 14% of previously treated pulmonary cases. Peru and Brazil reported half of all estimated MDR-TB cases.[6] Drug resistance surveillance should be strengthened in the Americas Region by performing resistance surveys in countries that lack reliable data, and through continuous resistance surveillance whenever possible.
In Cuba, as part of WHO’s post-2015 strategy, progress is being made towards TB elimination and continuous TB-resistance surveillance has been implemented. This activity, a vital element of the National TB Control Program (PNC), has revealed low MDR-TB prevalence. A study carried out in 2000–2009 showed 0.4% MDR-TB in new cases and 8.8% in previously treated cases,[7] while for these same categories, a 2010–2011 study revealed 1% and 10.4%, respectively, and found two XDR isolates (the first XDR detected in Cuba).[8] Although MDR-TB numbers detected in Cuba have been low, systematic and timely sensitivity testing of M. tuberculosis isolates from both new and previously treated cases constitutes a priority for PNC and the National Tuberculosis, Leprosy and Mycobacteria Reference and Research Laboratory (LNRITLM) of the Pedro Kourí Tropical Medicine Institute (IPK).[9]
At present a wide variety of methods, both phenotypic and genotypic, are used to test for antituberculosis drug sensitivity. Among these are methods based on solid medium (proportion method, or PM, in Löwenstein-Jensen or agar medium; resistance ratio method; and absolute concentration method) and in liquid culture medium. These methods are easily reproducible and their in vitro results correlate well with clinical course. The limitation of methods using solid medium is that they require several weeks of incubation due to M. tuberculosis’s slow growth, while the newer methods in liquid medium (BACTEC MGIT 960, Becton-Dickinson Diagnostics, USA and VersaTREK, Thermo Scientific, USA) provide results more quickly (8–12 days),[10] but are very expensive because they are based on automated systems, limiting their adoption in many countries.[11,12]
Understanding the molecular mechanisms of M. tuberculosis resistance has led to development of molecular tools, ranging from commercial systems (INNO-LiPA Rif.TB, INNO Genetics, Belgium, Genotype MTBDRplus and Genotype MTBDRsl, Hain Lifescience GmbH, Germany) to nucleic acid sequencing methods. Such methods are faster (not requiring bacterial culture, they can be done in a matter of hours) and highly sensitive, but their introduction in many countries is limited, since they require specific laboratory infrastructure and sophisticated equipment unavailable in countries with limited resources.[11,12]
Recently, WHO approved a group of alternative methods to investigate sensitivity to isoniazid and rifampicin; among these are the nitrate reductase assay (NRA), colorimetric assays using redox indicators, and the microscopic-observation drug-susceptibility assay.[13]
For many years surveillance of antituberculosis drug resistance in Cuba was carried out using PM in Löwenstein-Jensen medium. WHO approval in 2011 of several alternative methods for detection of isoniazid and rifampicin resistance permitted incorporation of new tools for Cuba’s resistance surveillance, which LRITLM now conducts using NRA and the molecular assay Genotype MTBDRplus to rapidly (≤5 hours) identify cases with resistance to isoniazid and rifampicin, while PM is reserved for sensitivity testing of second-line drugs and ethambutol.[9,13]
This study aimed to characterize antituberculosis drug resistance in isolates of M. tuberculosis from patients with bacteriologically confirmed pulmonary TB in Cuba in 2012 – 2014.
METHODS
We conducted an observational, cross-sectional study of 1472 isolates of M. tuberculosis from new cases and previously treated cases (relapses, therapeutic failures and losses to followup) of bacteriologically confirmed pulmonary TB, received at LNRITLM between January 1, 2012 and December 31, 2014. Isolates were received from Cuba’s 15 Provincial Hygiene, Epidemiology and Microbiology Centers, the Isle of Youth Municipal Center, the National Pulmonology Hospital and the Diagnostic Unit of the IPK LNRITLM. One isolate per patient was selected from among those received. Contaminated isolates and those containing fewer than 10 colonies were discarded. The final study sample consisted of 1068 isolates.
Each isolate was accompanied by a form completed by officials of PNC and the provincial laboratories, per PNC norms. For new and relapsed cases, isolates were obtained from cultured samples before starting treatment. For patients classified as treatment failures or lost to followup, isolates obtained from a sample taken prior to start of next treatment or within the first two weeks after its onset were analyzed.[9]
All isolates were studied for isoniazid and rifampicin sensitivity by NRA in Löwenstein-Jensen medium, using critical concentrations of 0.2 μg/mL and 40 μg/mL, respectively.[14] MDR isolates were studied by PM in Löwenstein-Jensen medium[15] for sensitivity to the following (critical concentrations for each in parentheses): streptomycin (4 μg/mL), ethambutol (2 μg/mL), ofloxacin (2 μg/mL), kanamycin (30 μg/mL), amikacin (30 μg/mL) and capreomycin (40 μg/mL), as well as to confirm sensitivity to isoniazid (0.2 μg/mL), and rifampicin (40 μg/mL).[16] All drugs were supplied by Sigma-Aldrich, Germany. Isolates monoresistant by NRA were analyzed for sensitivity to isoniazid and rifampicin using PM to confirm results.
The quality of each batch of culture medium used was verified for both NRA and PM, using M. tuberculosis strains of known sensitivity. For first-line drugs, M. tuberculosis strains ATCC 35822, ATCC 35820, ATCC 35837 and ATCC 35838, carriers of monoresistance to isoniazid, streptomycin, ethambutol and rifampicin, respectively, were used. For second-line drugs, strains with known resistance to ofloxacin, amikacin, kanamycin and capreomycin from Argentina’s Supranational Reference Laboratory were used, provided in 2009–2010 for external quality control of sensitivity tests. This quality control is carried out annually as part of the Global Project. M. tuberculosis strain H37Rv (ATCC 27294) sensitive to first- and second-line antituberculosis drugs was also used.[4]
Isolates identified as MDR by NRA were examined for mutations in the rpoB, katG, inhA, gyrA, rrs and embB genes using the commercial kits Genotype MTBDRplus and MTBDRsl, according to manufacturer’s instructions.[17,18] The Genotype MTBDRplus assay was used as an initial technique to test for resistance to izoniazid and rifampicin in isolates highly suspicious for MDR-TB.
Ethics This study was in PNC’s work plan and part of a project in the Ministry of Public Health’s program Advanced Techniques Applied to Diagnosis and Laboratory Investigation of M. tuberculosis and Other Mycobacteria (code: 131091). It was approved by IPK’s Research Ethics Committee (IEC-IPK-35-12). Handling of biological material was carried out by qualified personnel knowledgeable about biosafety standards for working with pathogenic microorganisms. All isolate manipulation was carried out in class II safety cabinets, which prevented external release of pathogenic microorganisms. Patient names and results were kept strictly confidential. Medical personnel responsible for patient care were informed of study results in a timely manner.
Analysis All information was processed in a Microsoft Excel database for Windows, and analysis was performed using EPIDAT version 3.1 for epidemiological analysis of tabular data. Results were expressed in absolute numbers and percentages. The Fisher exact test was used to compare proportions of resistance in new and previously treated cases, with a significance threshold of 0.05.
RESULTS
Of the 1068 M. tuberculosis isolates studied, 71 were nonviable, and in 7 cases it was impossible to determine whether or not there was prior history of antituberculosis drug treatment. Sensitivity analysis was thus restricted to 990 isolates, of which 836 were from new and 154 from previously treated patients (Table 1). Among the latter, there were 111 relapses, 11 treatment failures and 32 followup losses. Of the cases reported in 2012, 2013 and 2014, 58.8%, 74.5% and 64.5% of new cases and 78.3%, 87.7% and 92.6% of previously treated cases, respectively, were studied.
Table 1: Isoniazid and/or rifampicin sensitivity of M. tuberculosis isolates, Cuba, 2012–2014
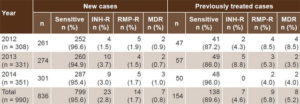
INH-R: isoniazid resistant (includes MDR) MDR: multidrug resistant RMP-R: rifampicin resistant (includes MDR)
Among new TB case isolates, 95.6% (799/836) were sensitive to isoniazid and rifampicin, and 0.8% (7/836) were identified as MDR. Among isolates from previously treated patients, 89.6% (138/154) were sensitive to isoniazid and rifampicin and 5.2% (8/154) were confirmed as MDR (Table 1), all relapses. Resistance to isoniazid was found in two patients whose treatment histories were not available (not included in Table 1).
The difference between the percentages of resistance in new (4.4%) and previously treated (10.4%) cases was statistically significant (p = 0.0043).
PM confirmed resistance in all isolates for which resistance to isoniazid and rifampicin was detected by NRA. Of 15 isolates identified as MDR, one became nonviable and thus could not be studied by other methods used in this study. Table 2 shows sensitivity patterns to other antituberculosis drugs in 14 viable isolates identified as MDR. Among the seven new cases, we identified one isolate sensitive to all drugs, two streptomycin-monoresistant isolates, three isolates resistant to streptomycin and ethambutol, and one resistant to all drugs except ofloxacin. Among the seven isolates of previously treated cases, two were monoresistant to streptomycin, and resistance to ofloxacin was found in another two, one of which was also resistant to capreomycin, kanamycin and ethambutol, showing XDR characteristics. The remaining three isolates were sensitive to all antibiotics. Amikacin resistance could not be assessed in 6 of the 14 cases.
Table 3 describes mutations found and resistance patterns identified using the Genotype MTBDRplus assay. Deviation from the wild-type rpoB gene was documented in all 14 isolates examined, corroborating phenotypic resistance to rifampicin found by NRA and PM. In 10 isolates, resistance was caused by the MUT3 (S531L) mutation. This mutation was found in six of the seven new case isolates. For the seven isolates from previously treated patients, rifampicin resistance was due to the MUT2B (H526D) mutation in two cases, and MUT3 (S531L) in four cases. Absence of hybridization with the WT2 probe, as well as the absence of the WT2 and WT3 bands, characterized rifampicin resistance of two isolates, one belonging to a previously treated case and the other to a new case. In neither of these cases was a mutation band present.
With respect to isoniazid, we observed deviation from the wild pattern of the katG gene in 10 isolates, but only 7 displayed the MUT1 (S315T1) mutation band (Table 3). With respect to the inhA gene, the MUT1 (C15T) band was present in a single isolate from a new case (Table 3). Use of the Genotype MTBDRplus assay allowed identification of MDR in 11 isolates (78.6%) (Table 3).
Finally, the use of the Genotype MTBDRsl assay revealed deviation from the wild pattern of the gyrA gene in two phenotypically ofloxacin resistant isolates. The WT3 band was not present in any of them. This finding was accompanied by the MUT3B band in one of the isolates. Concerning the rrs gene, the WT1 band was not present in either of the two isolates with phenotypic resistance to amikacin, kanamycin and capreomycin, and the A1401G (MUT1) mutation was present in both isolates. In three of the five isolates phenotypically resistant to ethambutol, mutations were found in the embB gene with the MUT1B (M306V) mutation.
Table 2: Sensitivity to antituberculosis drugs detected by proportions method, in MDR isolates of M. tuberculosis, Cuba, 2012–2014
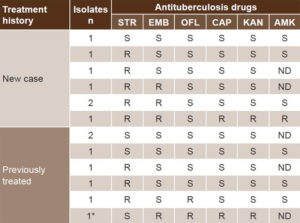
* Extensively drug-resistant AMK: amikacin CAP: capreomycin EMB: ethambutol KAN: kanamycin ND: not done OFL: ofloxacin R: resistant S: sensitive STR: streptomycin
Table 3: Mutations in rpoB, katG and inhA genes and sensitivity to rifampicin and isoniazid in MDR M. tuberculosis isolates, Cuba, 2012–2014
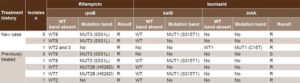
MDR: multidrug resistant MUT: mutation R: resistant S: sensitive WT: wild type
DISCUSSION
It is not surprising that PM confirmed isoniazid and rifampicin resistance in all isolates identified as resistant by NRA. Use of NRA at LNRITLM for several consecutive years showed excellent results for timely detection of resistance, not only to isoniazid and rifampicin, but also to streptomycin, ethambutol and second-line antituberculosis drugs.[8,19,20] Likewise, González (Colombia) recognizes the usefulness of NRA and reports a sensitivity of 91% and 92% for detection of resistance to isoniazid and rifampicin, respectively, as well as specificity of 100% for both drugs, compared to PM.[21]
MDR-TB rates detected in this study, both in new (0.8%) and previously treated cases (5.2%), are consistent with earlier LNRITLM reports of low circulating levels of MDR M. tuberculosis strains in Cuba.[7,8] The finding of new cases with rifampicin and isoniazid resistance constitutes a warning and points out the importance of searching for possible infection sources in such cases.
In the Americas, between 0% and 6.6% of new TB cases were MDR in 2012, the highest proportions reported in the Dominican Republic (6.6%), Ecuador (4.9%) and Peru (3.9%).[6] In countries lacking reliable data, surveillance of drug resistance should be carried out through surveys and, if possible, through continuous surveillance. The proportion of MDR-TB in previously treated cases was between 0% and 35%, with the highest percentages reported by Peru (35%) and Puerto Rico (33%).[6]
Global coverage for sensitivity testing of FQ and second-line injectable drugs is low. Only 23 of the 36 countries with high MDR-TB burden have reported data on resistance to second-line antituberculosis drugs. In 2012, 98 cases of XDR-TB were diagnosed in the Americas Region, a 26% increase over 2011; Brazil and Peru reported 16 and 67 cases, respectively.[6] To date, three cases of XDR-TB have been identified in Cuba, all in previously treated patients. One case was identified during this study, the two in 2011.[8] By 2014, at least one case of XDR-TB had been reported in 105 countries, with a relative frequency of 9.7% of MDR-TB cases.[2]
Among MDR-TB cases detected between 2010 and 2014 (including in our study), resistance to ofloxacin was found in four isolates of M. tuberculosis (two in this study) and in three of them, it was associated with resistance to second-line injectable drugs.[8]
The global panorama of M. tuberculosis resistance confirms the importance of systematically monitoring resistance to first- and second-line antituberculosis drugs. Ideally, sensitivity testing would be carried out before starting antituberculosis treatment, but in most countries, it is impossible to get phenotypic sensitivity study results in a timely manner, so patients receive empirical treatment. Molecular techniques can play an important role by rapidly assessing sensitivity and enabling early administration of optimal treatment regimens, consistent with resistance patterns of each strain.[22,23]
The findings of other authors, also using molecular procedures, coincide with ours in terms of mutations identified and their frequencies. Asencios (Peru) found high sensitivity and specificity of the Genotype MTBDRplus assay when compared to PM in agar. As in our study, S531L and S315T1 mutations were predominantly responsible for resistance to rifampicin and isoniazid, respectively.[24] Feliciano (Brazil) also found predominance of the S531L mutation among rifampicin-resistant isolates, while resistance to isoniazid was due to the S315T1 mutation in 50% of cases. In the remaining 50%, low resistance was found due to mutations in the inhA gene.[25]
Izoniazid resistance of the MTBDRplus genotype in 11 of the 14 isolates examined suggests the presence of infrequent mutations in the katG and inhA genes, or other molecular resistance mechanisms. Although the role of mutations in the kasA genes and the oxyR-ahpC and furA-katG intergene regions has not been fully elucidated so far, other studies have shown them in isoniazid-resistant isolates; regarding rifampicin, 96% of resistant strains present mutations in the 81 base pairs region of the rpoB gene.[26] However, there are reports of infrequent mutations outside this region that have also been associated with rifampicin resistance.[25] Our results suggest the need for research to elucidate the molecular mechanisms of resistance in Cuban isolates of M. tuberculosis.
In our study, the use of the Genotype MTBDRsl assay version 1.0 revealed an XDR pattern in a strain by finding mutations in the gyrA and rrs genes. This result was corroborated by demonstration of phenotypic resistance to ofloxacin, kanamycin and capreomycin using the reference technique.
Although we found no discordance between PM and the molecular method in sensitivity to second-line antituberculosis drugs, Theron’s 2014 meta-analysis reported that the Genotype MTBDRsl assay, version 1.0, failed to detect approximately one in five FQ resistance cases and one in four second-line injectable drug resistance cases. This assay rarely produces false positives for resistance.[27]
The recently released Genotype MTBDRsl assay, version 2.0, has advantages over version 1.0. In this version, in addition to the gyrA and rrs genes, mutations in the gyrB gene and in the promoter region of the eis gene are analyzed, increasing sensitivity in detecting FQ and second-line injectable drug resistance, particularly to kanamycin. This assay is a valuable tool, especially once WHO approved a shortened regimen for treatment of MDR-TB, since only patients with documented sensitivity to FQ and injectable drugs would be eligible.[23]
CONCLUSIONS
Our results suggest a low frequency of MDR M. tuberculosis in Cuba. Nonetheless, they warn of the need to continue improving surveillance of antituberculosis drug resistance by gradually increasing the number of isolates studied to ensure that all isolates from new and previously treated cases are tested for drug sensitivity. In addition, they demonstrate the importance of exhaustively searching for possible infection sources when resistant strains are encountered in new TB cases.






