INTRODUCTION
Anemia is a global health problem that affects all countries, with higher prevalence in low- and middle-income countries (LMIC). In 2011, children aged 6–59 months had the highest prevalence of anemia with an estimated 42.6% worldwide. The lowest prevalence was recorded in nonpregnant women, 29%, and within this population, it was lowest in the region of the Americas, 16.5%. Estimated prevalence of anemia in women of reproductive age was 23.9%–34.8%.[1] WHO defines anemia prevalence of 20%–39.9% as a moderate public health problem.[2]
Anemia has several causes, often coexisting, the main one identified as inadequate (quantitatively and qualitatively) intake of iron-rich foods. It is assumed that half of anemia is caused by iron deficiency; however, there are additional nutritional causes, including deficiency of other micronutrients such as folate, cobalamin, riboflavin or vitamin A.[2,3] Parasitism, malaria, tuberculosis and HIV are infectious causes of anemia. Cancer is one of the chronic conditions with concomitant anemia. Inherited or acquired errors of hemoglobin (Hb) synthesis, such as sickle cell disease, thalassemias, presence of HbE or disorders in the production or survival of erythrocytes, may also be causes of anemia in specific populations.[2,3]
Inflammation is a cause of anemia commonly seen in medical practice, but the size of its contribution is unknown in LMIC countries.[4] Inflammation is a reaction of an organism to bacterial or viral infections,[5] and an expression of chronic disease development, as well as obesity.[6] During the inflammatory process, interleukins are secreted that in turn stimulate liver secretion of the hormonal peptide, hepcidin. Hepcidin acts on intestinal cells, preventing iron absorption from food, and on the mononuclear phagocytic system, sequestering circulating iron.[7,8] In such cases, iron deficiency is due to both low dietary absorption and low bodily utilization.
Obesity is characterized by chronic, systemic, low-grade inflammation, with increased C-reactive protein (CRP), alpha 1-acid glycoprotein (AGP) and interleukin 6 (IL-6).[9–12] Therefore, overweight and obesity are related to iron deficiency and anemia through hepcidin secretion stimulated by high levels of IL-6 during inflammation.[10] In LMIC, there is a higher dual burden of malnutrition and infections, so assessment of the potential influence of these factors on serum ferritin (protein indicating iron nutritional status) is important in order to explain the prevalence of iron deficiency and its relation to anemia.[6]
Some authors associate iron deficiency anemia with H. pylori infection, because of the chronic gastritis it produces and consequent malabsorption of iron from food.[13,14] In LMIC, such as Cuba, colonization by H. pylori occurs during childhood and, if left untreated, remains a chronic infection into adulthood. A multicountry study of Latin American school children found an H. pylori prevalence of 47.7%, but not associated with anemia.[15] However, it is difficult to accurately estimate H. pylori infection’s contribution to development of anemia in women, since precision depends on the extent of infection, symptom severity and nutritional status.[13,14]
Proportions of anemia’s different causes vary among population groups and geographies.[1] Women of reproductive age are a risk group for anemia. This group exhibits factors that contribute to iron deficiency anemia, such as menstrual blood loss and scarce intake of iron-rich foods.[16] In turn, anemia in these women is a risk for pregnancy, childbirth and child development during the first year of life,[17] so deserves greater surveillance for improved maternal and child health.[3] WHO proposed the goal of reducing anemia by 50% by 2025 in women of reproductive age, compared to the baseline established in 1993–2005.[18]
By WHO definitions,[2] anemia in Cuba is considered a moderate public health problem, with a 21.6% prevalence in the third trimester of pregnancy.[17,19] The same prevalence was found between 2010 and 2013, in 4162 children aged 6–59 months.[20] A 2008 study of 1802 women aged 15–49 years in the eastern provinces of Cuba found anemia prevalence of 19.9%.[21] In 2015, there were 2,748,362 women in that age group in Cuba.[22]
We are unaware of any studies conducted in Cuba to assess risk factors associated with anemia in women of childbearing age. The objective of this study was to estimate the prevalence of anemia and iron deficiency in this age group and assess their relation to inflammation, overweight, central adiposity, H. pylori infection, and intake of iron-rich foods and enhancers of iron absorption.
METHODS
Study type and population An analytical, cross-sectional study was carried out from February through June 2014. Apparently healthy women, aged 18–40 years, living in 4 Havana municipalities (Playa, Cerro, Centro Habana and 10 de Octubre) were selected by two-stage cluster sampling.
Sample size was calculated using OpenEpi V3, according to the estimated population of women aged 20–39 years in Havana in 2011 (342,806), based on data from the National Bureau of Statistics and Information (ONEI),[23] and an estimated prevalence of H. pylori infection of 50% found in other studies in different population groups.[20] Maximum permissible type I error of 5%, 95% power and a design effect of 1 were accepted to obtain the minimum number of participants for primary prevalence estimates. The calculation yielded 384 women, to which 10% was added in anticipation of the possibility of nonresponse. The final sample size calculated was 422 women.
Participant selection was based on the pyramidal structure of Cuba’s health system, in which primary care is delivered at the municipal level. Each municipality is served by one or more polyclinics (staffed with primary care specialists), to which a variable number of family doctor-and-nurse offices (CMF) report.
Together the polyclinic and CMFs provide primary care to the polyclinic catchment area, known as the health area.[24] The two-stage cluster sampling included health areas as primary selection units and CMFs as secondary units.
Two health areas were selected in each municipality and within them three of the CMFs closest to the polyclinic, and which had adequate conditions for blood sample extraction. Due to the demographic similarity among health areas, this discretionary selection due to feasibility reasons does not introduce any apparent selection bias. At CMFs, the family physician and nurse invited all women within the established age range, and explained the objectives and content of the study.
The final number of women eligible to participate was 411, lower than the number initially calculated, but still higher than the 384 needed to ensure accuracy and reliability. Of these 411, 20 were eliminated by exclusion criteria listed below. The final sample had 391 participants.
Inclusion and exclusion criteria Women aged 18–40 years living in the selected health areas were included. Pregnant women, postpartum or having delivered less than six months before and women with sickle cell disease or those treated for hematological disorders were excluded. Also excluded were those experiencing an acute disease, or chronic diseases such as cancer, diabetes mellitus, hypertension, severe asthma, chronic obstructive pulmonary disease or renal failure.
Variables Table 1 summarizes the variables used and their indicators.
Procedures Biochemistry Anemia was assessed by Hb concentration in whole blood; iron deficiency by serum ferritin concentration; and inflammation by serum CRP (using the high sensitivity test), AGP and serum IL-6. Testing was carried out in the Nutritional Anemia Laboratory of the National Institute of Hygiene, Epidemiology and Microbiology (INHEM) by trained personnel, using reference material marketed and distributed in Cuba for quality control. The equipment used was calibrated by Cuba’s National Measurement Standards Research Institute.
Blood was extracted by antecubital venipuncture; 6 mL of whole blood was collected; 1 mL used for blood count and 5 mL for serum. Samples to determine Hb were analyzed on the day of extraction and serum was stored at -40 °C until being analyzed for ferritin and inflammation indicators.
Hb was determined with hematological analyzer ABX Micros 60 (Horiba, France). Ferritin and inflammation indicators determined by ELISA, using the Cloud-Clone Corp kit (USCN Life Science Inc., USA). Because not enough reagents were available for analyzing all samples, IL-6 was assessed in a discretionary subsample of 96 participants.
H. pylori infection The 13C–urea breath test was used to detect H. pylori infection,[28] Measurements were performed using the IRIS infrared isotope analyzer (Wagner Analysen Technik GmbH, Germany).
Anthropometrics Nutritional status was assessed by measurements of weight, height and minimum waist circumference according to the standardized protocol of the International Biological Program.[29] Body mass index (BMI) was calculated by the Quetelet formula: weight in kg/(height in m)2 (Table 1).[30,31]
Table 1: Variables, indicators and cutoff points

a 1–2 times weekly to daily b never to 1–3 times monthly BMI: body mass index DOB: delta over baseline (differential) of 13C/12C ratio of expired CO2
Food intake indicators Dietary assessment employed a 30-day recall food frequency questionnaire inquiring about foods rich in hemic (meat, viscera and blood products) and nonheme iron (eggs and legumes) foods one month prior to the study. Iron absorption enhancers, such as vegetables and fruits with significant vitamin C content, were assessed. The food groups assessed for associations with iron-deficiency were:
- meat products (pork liver, chicken liver, other organs, blood sausage, Italian sausage, ham, pork, red meats, poultry, fish, seafood, sources of hemic iron);
- eggs;
- beans (black, red or white);
- vegetables (lettuce, tomato, carrot, bell pepper, green beans, watercress, chard, cabbage, cucumber, spinach); and
- fruits (papaya, guava, mango, banana, pineapple).
Women who consumed fewer than four of these foods at least once a week were considered at risk for iron deficiency.
The number of foods in each group (meats, vegetables and fruits) eaten infrequently (1–2 times a week) to daily were counted for everyone. Consumption frequency was classified as:[33]
- never (not eaten during the entire period),
- very infrequent (1–3 times/month),
- infrequent (1–2 times/week),
- frequent (≥3 times/week; because of liver’s high iron content, at least once a week was considered frequent), and
- daily.
Consumption frequency was dichotomized as at least once a week (infrequent to daily) and less than once a week (never and very infrequent).
Data collection and analysis A database was prepared with data for all indicators of the variables studied. Statistical programs SPSS 20.0 and Epi Info 7.1.2.0 were used. Means and SD were calculated for indicators of biochemical and anthropometric variables. Biochemical indicators that were not normally distributed (such as Hb, ferritin, CRP, AGP and IL-6) were transformed logarithmically and their geometric mean and SD calculated. For variables that did not normalize even after transformation, the median and 25th and 75th percentiles were used.
Ferritin values were corrected for inflammation using the factors proposed by Thurnham for increases in CRP (0.77), AGP (0.75) or both (0.53).[34] Inflammation was assumed when at least one of CRP or AGP was elevated.
Overweight and obesity were grouped as excess body weight for analysis of anthropometric results. Classification based on waist circumference was used to assess central adiposity, considering that high adiposity includes increased and very increased (cardiovascular) risk cutoff points.[32]
The Mann-Whitney test was used to compare biochemical indicator means between groups infected with H. pylori, with a significance threshold of p <0.05.
Dependent variables were anemia and iron deficiency. Independent variables were inflammation, H. pylori infection, obesity, central adiposity, and consumption of iron-rich foods (with their respective indicators). For association analysis, variables were categorized into two or more levels and an odds ratio (OR) with 95% confidence interval was used.
Ethics Written informed consent was obtained from each participant. The study adhered to the principles of the Declaration of Helsinki[35] and was approved by the ethics committees of the Center for Nutrition and Food Hygiene and INHEM.
RESULTS
Of the women studied, 24.6% (95% CI 20.3–28.9) (96/391) had anemia: mild in 62.5% (60/96) and moderate in 37.5% (36/96). Iron deficiency was found in 68% (266/391) of participants and in 82.3% (79/96) of those with anemia. There was a significant association between iron deficiency and anemia (OR 2.68, 95% CI 1.51–4.77).
Table 2 shows the medians, 25th and 75th percentiles, and value ranges for Hb and ferritin, both with asymmetric distributions even after logarithmic transformation. More than 50% of participants had ferritin values indicating iron deficit. The table also displays geometric means, quartiles and value ranges for the other biochemical and anthropometric indicators (the latter with means and SD).
Prevalence of inflammation was 8.4% (33/391) and 19.9% (78/391), assessed by CRP and AGP, respectively. Some 73.1% (286/391) had normal values of both CRP and AGP; 25.3% (99/391) had one of these indicators elevated, and 1.5% (6/391) had both.
Nutritional status based on BMI could not be assessed in 3 women who did not appear for physical measurements. In the remaining 388, normal nutritional status predominated in 53.3% (207/388). However, 38.7% (150/388) had excess body weight and 26.7% (101/378), increased central adiposity; 8% (31/388) were underweight. It was impossible to assess central adiposity in 10 women: 8 aged 19 years because there are no reference values for their age group, and 2 who appeared for measurement when the needed specialist was absent.
Prevalence of H. pylori infection was 47.1% (184/391). No significant differences were found between groups with and without infection in mean Hb (p = 0.939), ferritin (p = 0.180), CRP (p = 0.313), AGP (p = 0.597) or IL-6 (p = 0.671) (Table 3).
Table 4 shows positivity for inflammation markers (CRP and AGP), independently and in combination, in women with and without risk factors (H. pylori infection, excess body weight and central adiposity). It also displays ORs (with 95% confidence intervals) reflecting associations between risk factors and inflammation markers. Excess body weight and central adiposity exhibited significant associations with CRP; there were no significant associations with AGP.
Table 5 shows frequency of food consumption in two complementary categories, at least once a week and less than once a week. The most frequently consumed meat products were poultry (81.9%), ham (64.4%) and pork (63.6%); viscera were consumed at least weekly by fewer than 10% of respondents. Among nonheme iron sources, the most frequently consumed were eggs and beans, consumed at least weekly by 75.8% and 61.1%, respectively. Tomatoes were the most frequently consumed vegetable, eaten by 82.2% at least weekly; <40% of respondents consumed the remaining vegetables at least weekly. Bananas were eaten at least weekly by 61.8% of women, but less than half (in some cases less than a third) ate any of the other fruits that frequently.
Table 2: Biochemical and anthropometric indicators to assess anemia, iron defi ciency, infl ammation and nutritional status in women of childbearing age, Cuba, 2014

*corrected for inflammation AGP: alpha 1-acid glycoprotein BMI: Body mass index CRP: C-reactive protein Hb: hemoglobin IL-6: interleukin-6 P25: 25th percentile P75: 75th percentile
Table 3: Biochemical indicators in women of childbearing age with and without H. pylori infection, Cuba, 2014 (n = 391)
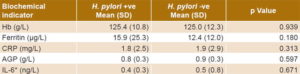
*n = 96 AGP: alpha 1-acid glycoprotein CRP: C-reactive protein Hb: hemoglobin IL-6: interleukin-6
Table 4: Anemia risk factor prevalence and association with inflammation markers in women of childbearing age, Cuba, 2014
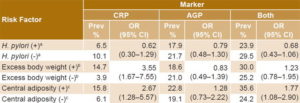
an = 391 bn = 388 cn = 378 AGP: alpha 1-acid glycoprotein CRP: C-reactive protein
Analysis of the association between inflammation and iron deficiency resulted in OR 1.72 (95% CI 1.03–2.87), while the association between inflammation and anemia was significant but in the opposite direction: OR 0.55 (95% CI 0.31–0.98). No association was found between H. pylori and anemia (OR 0.94, 95% CI 0.59–1.49) or low ferritin (OR 0.75, 95% CI 0.49–1.14). Excess body weight was not positively associated with iron deficiency or anemia, but central adiposity had significant protective associations in both cases (OR 0.54, 95% CI 0.33–0.89 and OR 0.44, 95% CI 0.24–0.82, respectively). No significant associations were found between consumption frequency of any food and anemia or iron deficiency (all OR confidence intervals included unity) (Table 6).
Table 5: Consumption of iron-rich foods and absorption enhancers in women of childbearing age, Cuba, 2014 (n = 343)
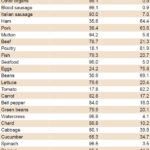
Table 6: Association of inflammation, H. pylori infection, excess body weight and obesity with anemia and iron deficiency in women of childbearing age, Cuba, 2014
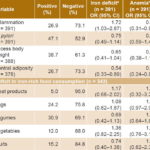
a ferritin <15 μg/L (68% of sample) b hemoglobin <120 g/L (24.6% of sample)
DISCUSSION
The anemia prevalence we found is consistent with earlier estimates in Cuba[2,17] and comparable to that of some other countries in the Americas and the Caribbean Region such as Guatemala, Brazil, Dominican Republic and Bolivia, where prevalence ranges from 21.4% to 38.3%.[36] However, other studies have found lower prevalence. For example, anemia prevalence was 16.7% in a national sample of women aged 19–35 years in Mexico in 2006,[37] and a study in Chile from 1981–2010 found 9.6% in the first year and 10% in 2010.[38] Although our study was carried out in only 4 municipalities of Havana and is not representative of the city or the country, Cuba’s relative demographic, cultural and socioeconomic homogeneity suggests that our estimates should not differ greatly from those derived from a more comprehensive sampling.
In 2014, Kassebaum published a systematic analysis of the global burden of anemia from 1990 to 2010 and found that in the Caribbean region, iron deficiency was the leading cause of anemia in both sexes. The following causes among women, in order of importance, were sickle cell disease, Ancylostoma spp. infection and uterine fibroid.[39]
In our study population, iron deficiency was an important factor in anemia and was found to be related to inflammation. In contrast, a negative risk association was observed between inflammation and anemia.
Current evidence suggests an association between inflammation and anemia, although the strength of the association appears to be lower than that found for specific micronutrients, such as iron, mainly in preschool groups.[7] This was a cross-sectional study and timing might have affected findings. Erythrocytes have an average life span of 120 days, and anemia is the last stage of iron deficiency, CRP and AGP indicate subclinical inflammation. CRP peaks in circulation 48–72 hours after an inflammatory injury and then declines, and AGP peaks in 3–5 days,[27] so the effects of inflammation on anemia cannot be observed in early stages (unlike ferritin, which is the first indicator of iron depletion). A physiological response to inflammation can appear quickly, allowing the body to remain healthy and in homeostasis, preventing mobilization of iron from tissues and using the scarce iron absorbed directly from food for Hb synthesis, before developing anemia. Median Hb found in this study is close to the cutoff point for anemia, so that a precarious iron balance may exist in the body preceding development of anemia a short time afterwards. This may also indicate the influence of other factors on iron deficiency and anemia, such as heavy menstrual periods.[40,41]
Assessment of nutritional status showed that over one third of participants had excess body weight. This proportion is similar to those found in Mexico (35.5%)[37] and Colombia (42.3%),[42] but lower than that detected in Cuba’s Third National Survey on Risk Factors and Chronic Diseases (ENFRENT III, 2010–2011), which found excess body weight in 48.3% of women aged ≥15 years.[43] Studies in LMIC have revealed an association between anemia and obesity,[36,44,45] which may be explained by the inflammatory action of adipose tissue. However, we found no such association. On the contrary, a trend to lower prevalence of anemia and iron deficiency with increased BMI was observed, similar to findings by Kordas,[42] Qin[46] and Wendt,[11] in which women with excess body weight were less likely to have anemia or iron deficiency than those with normal weight. One explanation may be that overweight women have generally ingested more food, including those rich in iron and absorption enhancers.[46]
Another aspect to consider is that increased central adiposity and excess body weight were associated with CRP-expressed inflammation more strongly than with AGP, so when assessed jointly, the level of the association is weakened.
BMI has limitations as an indicator of adiposity, and waist circumference may be a better marker of total body fat composition. Obesity with normal weight has been defined as excess fat with normal BMI, and this condition has been associated with early inflammation. Marqués Vidal found fat percentage above the 66th percentile with normal CRP values in 5.4% of a group of women with BMI <25.[47] Cuba’s ENFRENT III found 51.5% of women aged ≥15 years had increased risk and very increased risk of central adiposity, as indicated by waist circumference much higher than the one found in this study but did not assess biochemical indicators of inflammation.[43]
H. pylori infection was frequent in our study population, but not associated with iron deficiency or anemia. The sample did not include women with symptoms of digestive disorders, so it may be that asymptomatic H. pylori colonization (i.e., without ulcers or gastritis) does not produce iron absorption disorders and anemia, nor increase inflammation indicators. Previous studies in other Latin American countries (Argentina, Brazil, Mexico, Bolivia, and Venezuela) also found no associations between H. pylori and ferritin or soluble transferrin receptor concentrations, leading authors to conclude that there is no evidence in the region supporting the hypothesis that H. pylori infection contributes to anemia or iron deficiency.[15]
The food frequency questionnaire is useful for estimating usual food intake. The diet consumed by women in our study reflects lack of diversity in food selection, which was also found in previous studies in groups of children and persons aged >15 years, with a similar pattern of behavior throughout Cuba and with low intake of A and B-complex vitamins and iron, among other nutrients.[48,49]
The absence of a significant association between food consumption frequency and either iron deficiency or anemia may in part be due to the multiplicity of factors that affect nutrient absorption and digestion, which also depend on prior nutritional status.[50] Thus, dietary intake is a limited indicator of individual nutritional status. Furthermore, the food frequency questionnaire does not ask about quantities, so we could not estimate actual amounts of iron ingested.
The US National Health and Nutrition Examination Survey also used the 30-day food frequency questionnaire, in nonpregnant Mexican-American and non-Hispanic white women aged 12–39 years (1988–1994), and found that, contrary to expectations, dietary intake patterns of iron-containing foods did not explain the study group’s low iron levels.[51]
Some methodological differences must be considered when comparing our results with others, particularly the use of different cutoff points. While we used Thurnham and WHO’s recommended CRP cutoff points,[27,34] especially to correct for ferritin levels, other authors have assessed inflammation using different cutoff points for example, that recommended by the American Heart Association (CRP >3 mg/L) for assessment of obesity and adiposity.[11,52] Gartner’s study of 811 Moroccan women used CRP >2 mg/L relative to ferritin value increases;[53] and Lourenço used 1 mg/L.[54] The question of optimal cutoff points is one that should be addressed in future population studies.
One study limitation is its cross-sectional design, which does not allow causal inference, because it could not establish chronological order of obesity, inflammation and H. pylori with respect to anemia and iron deficit. Another was the practical infeasibility of studying other nutritional factors for anemia and iron deficiency, thus limiting our ability to control for potential confounders. One such variable is menorrhagia, which should be included in future research.
The study did not confirm the expectation of a positive association between anemia and inflammation (measured by two of its markers), central adiposity or excess body weight. The most plausible explanation is the so-called “collision bias” that occurs when assessing association between two variables with a common cause.[55] If, for example, there are factors underlying inflammation, adiposity and excess body weight that also have a causal relationship to anemia, measurement of the association between anemia and each of the three risk factors would be biased, even in the opposite direction, which seems to have happened in this study.
Despite its design limitations and the inability to control some variables and risk factors, this study provides information unavailable thus far in Cuba for an important population group: women of childbearing age.
CONCLUSIONS
Anemia was shown to be a moderate public health problem in the studied group, but with iron deficiency present in two thirds of participants and associated with anemia. Risk factors for anemia and iron deficiency, such as menorrhagia and bacterial or viral infections, should be assessed in women of childbearing age, to support interventions needed to reduce risks in pregnancy and childbirth.
ACKNOWLEDGMENTS
The authors would like to thank Iris Cortina Mena MD (Provincial Hygiene Epidemiology and Microbiology Center, Havana), Ahindris Calzadilla Cámbara MD, family physician (INHEM), Zuleimi Guerra Montané (Department of Biochemistry and Physiology, INHEM) and Cornelia Loechl, PhD (International Atomic Energy Organization, Vienna) for their support in implementing the study, including sample collection.
FUNDING
The study was funded by Cuba’s Ministry of Public Health (National Project 14-1-098) and by the International Atomic Energy Organization (IAEA-Coordinated Research Project E4.30.25).









