INTRODUCTION
The famous case of Theresa Marie Schiavo raised new controversies regarding end-of-life decisions for patients in persistent vegetative state (PVS), stirring a contentious public debate, and pitting her siblings and parents against her spouse over continuing the use of a feeding tube to keep her alive. The debate dominated US national discourse and was carried out by the media, the courts, the Florida legislature, the Florida Governor, the US Congress, even reaching the President of United States. Her case and those of others receiving wide media attention—including Karen Ann Quinlan and Nancy Cruzan in the United States and Tony Bland in the UK—have obliged neurologists and other neuroscientists to propose reliable diagnostic guidelines for testing brain function in altered states of consciousness. In fact, management of PVS and minimally conscious state (MCS) cases is one of the most difficult medical, ethical and social dilemmas faced by medicine today.[1]
The term PVS was coined by Jennett and Plum in 1972 to describe wakeful patients with apparent loss of awareness,[2] with home health care providers and family cannot establish any direct communication. Hence, by definition, subjects in PVS, with no recognizable behavioral responses to external stimuli, are considered isolated from the outside world. From this observers have inferred—wrongly, in our opinion—that these patientes therefore cannot experience pain or suffering.[3]
PVS is a condition in which a patient with preserved sleep-wake cycles, respiration, digestion and thermoregulation has an apparent loss of awareness of self and the environment. After 35 years, The European Task Force on Disorders of Consciousness proposed a new term for this syndrome—unresponsive wakefulness syndrome (UWS)—avoiding the deprecatory term vegetative state.[4] We fully agree with this proposal: the association with plant life is too vivid in both Spanish and English not to have a pejorative connotation, as any physician can attest who has had to explain PVS to patients’ family members.[5–12]
Giacino et al. first suggested the term minimally conscious state, proposing that “to make the diagnosis of MCS, limited but clearly discernible evidence of self or environmental awareness must be demonstrated on a reproducible or sustained basis.”[13] We submitted a response to that paper, proposing the use of the term minimally aware state instead of minimally conscious state, because the two components of consciousness are arousal and awareness, and the main difference between PVS and MCS is a partial recovery of awareness.[1]
We heartily support the effort to find more appropriate terms to describe patients with disorders of consciousness.[4,14–18] We also are aware of the need for change in the terminology of non–English-speaking societies, to avoid pejorative medical terms.[11] However, in this paper we will continue to refer to PVS and MCS, given that consensus on new terminology has not yet been reached.
The Cuban Group for study of Disorders of Consciousness is developing several research protocols to search for preserved residual brain and autonomic functions in PVS and MCS cases. We have shown recognition of a mother’s voice with an emotional content—indicating high-level residual linguistic processing and brain activation—after zolpidem administration, in spite of the patient’s apparent inability to communicate.[5–12]
In this paper we will refute the concept that, by definition, persons in PVS with no recognizable behavioral responses to external stimuli are isolated from the outside world.
ANATOMICAL AND FUNCTIONAL CONNECTIVITY IN PVS
According to Kinney et al., PVS denotes a “locked-out” syndrome because “the cerebral cortex is disconnected from the external world and all awareness of the external world is lost.”[19] They suggest that loss of awareness in PVS is caused by three main patterns: widespread and bilateral lesions of the cerebral cortex, diffuse damage of intra- and subcortical connections in the cerebral hemispheres’ white matter, and necrosis of the thalamus. In many PVS patients, the lesions are a mix of the above-mentioned neuropathologic findings.[11,19]
Hence, detailed description of lesion location in these cases is critical. Magnetic resonance imaging (MRI) is the most powerful tool for examining neuropathological lesions in PVS patients.[11]
We are running protocols to evaluate neuropathology in PVS and MCS cases, studying patients using T1 MRI with 1 mm slices and 3D reconstruction of brain images.[1,6,7,10,11,20] This method allows detailed visualization of pathological lesions (Figure 1).
Figure 1: 3D Anatomical reconstruction of brainstems of normal subject and a PVS patient
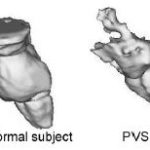
3D anatomical reconstruction with T1 MRI shows massive destruction of the upper brainstem in a PVS patient.
Anatomical connectivity It is widely accepted that normal brain function depends on activity synchronization within distributed brain networks and that disruption of those sets of connections may explain brain dysfunction.[21,22] Magnetic resonance diffusion tensor imaging allows assessment of brain white matter anatomic connectivity (tractography) to characterize specific white matter lesions such as atrophy and diffuse axonal injury. The direction of water diffusion in myelinated fibers matches the direction of white matter tracts within the brain. Hence, the diffusion constant and fractional anisotropy are related to the density, diameter and geometry of myelinated fibers.[10,23] We are using this technique to assess anatomical connectivity in PVS and MCS patients.[11] This is crucial for explaining and understanding the pathophysiology of consciousness disturbances in these cases.
We used fractional anisotropy to assess a 15-year-old girl with sickle cell disease who developed important cognitive impairment due to multiple strokes and had been diagnosed as in PVS. Nonetheless, when she was later admitted to the Neurology and Neurosurgery Institute with inconsistent but clearly demonstrable behavioral evidence of awareness, we changed our diagnosis to MCS. Fractional anisotropy in combination with MRI showed preservation of anatomical connectivity among posterior brain regions, which were also connected with remaining frontal cortex islands. These remaining cortical regions were also connected with the thalami. We concluded that white matter connectivity among posterior and frontal cortical regions and the thalami and cerebral blood flow preservation in the cortical areas may explain recovery of minimum awareness despite sizeable anatomical brain lesions.[10] A possible axonal rewiring mechanism could explain late recovery in these cases, as reported by other authors.[10,23]
We have described two main patterns of white matter disruption in PVS cases. In diffuse lesions, such as postanoxic encephalopathy, remaining tracts surround the dilated ventricles (Figure 2). In focal lesions, such as intracerebral hemorrhage, MRI tractography shows focal tract disruption, resembling scissor cuts, as arrow indicates in Figure 3.[11]
PROTON MAGNETIC RESONANCE SPECTROSCOPY IN PERSISTENT VEGETATIVE AND MINIMALLY CONSCIOUS STATES
Proton magnetic resonance spectroscopy (1H-MRS) is a powerful tool to assess biochemical changes in vivo in nervous system diseases. N-acetyl aspartate (NAA) content quantifies neuronal integrity, while choline concentration reflects membrane turnover and creatine is related to energy dependent systems. A decrease in NAA concentration is a sign of neuronal loss or dysfunction.[1]
Figure 2: MRI T1 images and MRI tractography in a PVS patient with postanoxic encephalopathy after near drowning
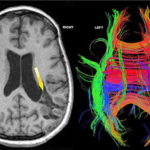
MRI T1 image (left) shows dilated ventricles and hydrocephalus in a PVS case. MRI tractography (right) shows white matter tracts surrounding the dilated ventricles.
Figure 3: MRI T1 images and MRI tractography in PVS patient with intracerebral hemorrhage
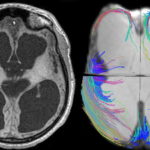
MRI T1 image (left) shows focal lesions (arrow) in right parietal hemisphere, due to intracerebral hemorrhage (blood already reabsorbed). MRI tractography (right) shows focal white local tract disruption, resembling scissor cuts.
We recently described metabolic changes assessed by MRS in two patients who evolved from PVS to MCS and two others who remained in PVS.[10] We believe the most important finding in our studies was an increased NAA/creatine ratio in the cortex in both cases of transition from PVS to MCS, while the pair who remained in PVS showed lower values of this MRS measure. Hence, MRS may provide a useful neurobiological marker to follow up cognitive recovery in PVS patients transitioning to MCS.[1,10]
RECOGNITION OF MOTHER’S VOICE WITH AN EMOTIONAL CONTENT IN A PVS PATIENT
One crucial question is whether or not patients in PVS can recognize relatives’ voices. We are running protocols to assess if there is significant differential brain activation in response to a mother’s voice, compared with that when voices of unknown women are presented (sham voices). We recently reported experience with an eight-year-old boy who after a near drowning remained in PVS for four years before the study. We investigated whether there was significant differential brain activation in response to hearing his mother’s vs. a sham voice, using quantitative electric tomography, which combines anatomical information about the brain from MRI with EEG patterns to estimate sources of brain activation. We found EEG activation indicating high-level residual linguistic processing in this patient meeting clinical criteria for PVS.[1,12]
We also assessed autonomic responses to mother’s voice as indicated by heart rate variability (HRV), applying time-varying spectral analysis to sequential series of electrocardiogram R–R intervals. We found that during the sham voice experimental condition there was a significant increase in the HRV very low frequency (VLF) band and a significant decrease in the HRV high frequency (HF) band, indicating sympathetic triggering and a reduction of parasympathetic activity. Nonetheless, during the mother’s voice condition, an increase in the VLF band was observed, combined with a significant recovery of the HF band, revealing both sympathetic and parasympathetic activation. This can be explained on the basis that the sham voice induces an arousal characterized by sympathetic activation, while the mother’s voice induced arousal, characterized by both sympathetic and parasympathetic activity, probably related to a positive emotional reaction during this experimental condition.[5]
PHARMACOLOGICAL INTERVENTION WITH ZOLPIDEM IN PVS CASES
Molecular and neural mediators may indirectly help to enhance the phenomenon known as neural synaptic plasticity.[24–26] Hence, several specific pharmacologic approaches to assisting brain function recovery in these cases have been investigated.[27–29]
Several reports have been published over recent years about the paradoxical arousal effect of zolpidem tartrate, a highly selective nonbenzodiazepine gamma aminobutyric acid agonist, which acts as a sedative in normal subjects.[30–35] This astonishing effect was first described by Clauss et al. as an accidental discovery: after zolpidem administration in a patient who had been in PVS for more than three years following a motor vehicle crash, he awoke and could recognize and greet his mother for the first time since his injury.[36]
Clauss’s report led several authors to explore zolpidem’s effects in patients with PVS, MCS, ischemic stroke, brain injury, hypoxic encephalopathy and other neurological disorders, using neuroimaging techniques to assess brain function.[27–35] A number of authors have subsequently described transient but dramatic improvement in motor and language status in some PVS patients, some of whom even recovered a degree of spontaneous movement and were able to walk.[27–29] Several of these clinical improvements have been correlated with improvement in metabolic and electrical brain function. Brefel-Courbon et al. used positron emission tomography to assess a patient with hypoxic encephalopathy in PVS and demonstrated a marked increment in anterior forebrain metabolism with zolpidem administration.[35]
We recently reported a PVS case in which marked behavioral signs of general arousal were observed, associated with significant autonomic, EEG and funcional MRI activation after administration of a single 10-mg dose of zolpidem.[5,6] We studied a female patient (Y.O.R.) aged 21 years with basilar artery syndrome secondary to a stroke, who had been in PVS for five years. MRI revealed destruction of the rostral pons, the mesencephalon and both thalami. Y.O.R. showed circadian wakefulness, although she kept her eyes closed most of the time. With written informed consent from her parents, 10-mg of zolpidem was administered via percutaneous endoscopic gastrostomy.[6] After administration of zolpidem, Y.O.R. began to open and close her eyes, continuing to do so for some 15–20 minutes until her eyes remained open from minute 25 to minute 46. She yawned spontaneously at minutes 27, 28, 29, 31, 37 and 39.
In this case, the relative power spectral density (PSD) in the delta band constituted 89–96% of total power density for the EEG spectra in the different EEG leads and mean frequency was 1.34 Hz. Over higher frequency ranges (theta, alpha and beta), EEG activity was negligible, so quantitative analysis focused on possible changes in the lower EEG frequencies (0.146–2 Hz). During the post-zolpidem period, some modulation was observed in delta wave amplitude and morphology, coinciding with spontaneous eye movements and yawning, but quantitative analysis did not reveal statistically significant differences compared to the control EEG. Post-yawning averaged EEG spectra showed a significant (p < 0.05) increment of delta PSD, considered in normalized units (%) in all EEG leads, in comparison with the pre-yawning EEG segments of the same duration of 40.96 seconds, and a corresponding reciprocal significant decrease of PSD of EEG activity in the infra-slow EEG frequency band in post-yawning averaged spectra.
Zolpidem’s effect on autonomic function was also assessed by examining HRV. Dynamics of low frequency (LF), HF and VLF bands considering normalized units (%) showed a significant increment (p < 0.05) from minutes 6 to16 in HF power and a corresponding reciprocal reduction in LF band power. Similar changes were observed from minutes 20 to 23, 28 to 30, 53 to 54, and finally from 58 to 60. From minutes 24 to 27, a reversal of the previously described situation was observed, characterized by increased power in the LF band with reciprocal reduction in the HF band. This pattern reappeared from minutes 37 to 43. Power values in the VLF band markedly increased its control values from minutes 25 to 35, and later, although not as a peak, remained significantly increased until minute 47.
Autonomic dynamics after zolpidem in patient Y.O.R., as described earlier, clearly showed periods of both parasympathetic and sympathetic cardiovascular predominance. Most interestingly, sympathetic cardiovascular predominance coincided with yawns and behavioral signs of arousal.
Although this was only a single case report, our findings showing a clear relationship between behavioral signs of arousal and specific changes in autonomic cardiovascular regulation, as well as a minimal but significant shift to higher EEG frequencies, demonstrate the importance of assessing brain-heart connections in explaining the paradoxical effect of zolpidem in PVS and MCS patients.
MRI BOLD SIGNAL FOR ASSESSING BRAIN METABOLIC CHANGES AFTER ADMINISTRATION OF ZOLPIDEM IN A PVS PATIENT
Functional MRI based on blood oxygen level-dependent (BOLD) signal is related to a variety of physiological parameters as well as cerebral blood flow. Magnitude of BOLD change due to elevated neuronal activity is determined by decreased susceptibility effects resulting from local increases in oxygenated hemoglobin.[37]
We also studied patient Y.O.R. using 1H-MRS and BOLD signal, before and after zolpidem administration. Significantly increased BOLD signals were localized in the left frontal superior cortex, bilateral cingulate areas, left thalamus and right head of the caudate nucleus. Transient activation was observed in the frontal cortex, comprising portions of anterior cingulate, medial and orbito-frontal cortices. Additionally, we found marked pharmacological activation in the sensory-motor cortex one hour after zolpidem intake. Statistically significant linear correlations of BOLD signal changes were found with primary concentrations of glutamate in the right frontal cortex. We hypothesized that when zolpidem attaches to neurodormant cells’ modified GABA receptors, dormancy is switched off, inducing brain activation. This might explain the significant correlations of BOLD signal changes and 1H-MRS metabolites in our patient. We concluded that 1H-MRS and BOLD signal assessment may help study neurovascular coupling in PVS cases after zolpidem administration. Although this was a single case report, our observations led us to recommend applying these methods in a series of PVS and MCS patients.[5]
CONCLUSIONS
Development of interventions for treatment and rehabilitation of patients with disorders of consciousness is a crucial challenge for current and future generations of neuroscientists. The management of PVS patients is an extremely difficult task for relatives and society in general, and these cases are usually considered hopeless. Although current treatments promoting recovery in such cases are extraordinarily limited, our findings suggest new medical, ethical and practical implications for the diagnosis and management of PVS patients.
Our observations have led us to differ with the current concept that, by definition, patients in PVS with no recognizable behavioral responses to external stimuli are isolated from the outside world. We have research under way to address the assumption that they cannot therefore experience pain or suffering.
The Cuban Group for Study of Disorders of Consciousness is now running a trial to assess the dynamics of clinical, behavioral, autonomic and other vital physiological índices after administration of single 10-mg doses of zolpidem in a group of PVS patients. This investigation will allow us to determine which patients respond favorably to zolpidem administration. Similar trials will be developed in the near future with other drugs that may indirectly contribute to restoring brain function in patients with such disorders of consciousness..






