ABSTRACT
Introduction There is growing interest in beating heart cardiac surgery (mainly myocardial revascularization) without aortic cross-clamping and, if possible, without the use of a cardiopulmonary bypass (CPB) pump, since better results can be obtained with this procedure than with conventional myocardial protection techniques using cardioplegic solutions. This led us to considerer mitral valve replacement (MVR) with beating heart and continuous coronary perfusion as a surgical option at the Cardiology and Cardiovascular Surgery Institute (ICCCV) in Havana, Cuba.
Objective To assess the safety and potential benefits of beating heart MVR with continuous coronary perfusion compared to the conventional cardioplegic arrested heart MVR procedure.
Methods A randomized, controlled intervention study was conducted with a sample of 64 patients referred to the ICCCV for isolated MVR between January 2001 and December 2002. Patients were randomly divided into 2 groups: control group A and study group B. Each group received a specific myocardial protection technique during surgery. Group A underwent MVR using the arrested heart technique with administration of a cold crystalloid cardioplegic solution and with moderately hypothermic CPB. Group B underwent MVR using the beating heart technique with normothermic CPB and continuous coronary perfusion. The following variables were assessed: serum enzyme (CK and CK-MB) and lactate concentrations; duration of aortic cross clamping, CPB, mechanical ventilation support, drainage, postoperative bleeding, stay in the surgical intensive care unit (SICU), and total operation time; amount of blood lost, blood adminstered, and postoperative complications. Quantitative variables were determined using Wilcoxon-Mann-Whitney and Student’s t-tests.
Results Differences between the two techniques were not found to be statistically significant, which suggests that both are equally safe. However, the differences found are clinically important and favor the beating heart technique, since patients who underwent beating heart MVR had lower serum concentrations of total CK, CK-MB and lactate; less total blood loss, and less need for transfusion. They also required less time on mechanical ventilation support in the SICU, spent fewer days in the hospital, and presented fewer postoperative complications compared to patients who underwent arrested heart MVR.
Conclusion The beating heart technique with continuous coronary perfusion proved to be as safe as the conventional arrested heart technique with cardioplegic solutions for MVR surgery in patients with low surgical risk. This procedure is recommended as an alternative method of myocardial protection for this type of surgery in Cuba and may be considered as an option in other limited-resource settings.
Keywords Heart surgery, cardiac surgery procedure, mitral valve, beating heart, induced heart arrest
INTRODUCTION
Since the advent of cardiopulmonary bypass (CPB), and with it open-heart surgery, the main concern of surgical teams has been finding the best means of protecting the heart from the harmful effects of myocardial ischemia.[1] A variety of myocardial protection methods have been devised and used during these procedures; however, none has proved ideal, achieving optimal results under all clinical circumstances.[2]
The myocardial protection technique most commonly used in heart surgery today is administration of a cardioplegic solution that quickly stops mechanical and electrical heart activity in diastole (heart arrest), thereby creating a motionless and bloodless surgical field. [2] These conditions are achieved at the expense of inducing a variable period of global myocardial ischemia, which can be well tolerated by the heart without evidence of cellular damage or, if the myocardium is already predisposed, may cause irreversible damage with necrosis and cell death due to reduced perfusion or increased metabolic demand.[1] Furthermore, contact with artificial surfaces during the blood’s passage through the CPB pump causes a diffuse inflammatory response that affects multiple organs, principally the heart itself, as well as the liver, lungs, central nervous system, kidneys and gastrointestinal tract.[2]
Interest in beating heart cardiac surgery without aortic cross clamping and, whenever possible, without CPB, has increased in recent years as a means of avoiding the adverse effects of CPB. Myocardial revascularization with coronary artery bypass grafting has been one of the major beneficiaries of this technique. [3-6]
In general, authors have reported better results with the beating heart technique than with the arrested heart technique in terms of duration of mechanical ventilation support, need for transfusion, incidence of low postoperative output, incidence of postoperative arrhythmia, length of hospital stay, and procedure costs.[4,5,7]
Interest in beating heart surgery intensified with the development and popularization of minimally invasive surgical procedures in developed countries, and is now being used by a growing number of cardiac teams throughout the world.[8-10] Minimally invasive heart surgery, performed through limited incisions and, in most cases, without CPB, is considered the leading technological innovation of the last decade in the cardiac surgery field.[9] This procedure is characterized by the development of new tools and techniques that can be used to perform many different operations through smaller entry points and video-assisted surgery without CPB, or with CPB and peripheral vessel cannulation.[10] This surgical approach helps avoid systemic inflammatory response resulting from the use of CPB and helps reduce surgical trauma, which, in turn, reduces morbidity and speeds postoperative recovery.[9,10]
In Cuba, a developing country with a universal health care system, minimally invasive off-pump beating heart coronary artery bypass surgery has been performed for over ten years, resulting in a significant reduction in ischemic damage to the myocardium, fewer postoperative complications and fewer postoperative blood transfusions compared to procedures carried out using the conventional arrested heart technique and CPB.[11]
Given the satisfactory results obtained with this approach, and considering that other open heart surgeries using the beating heart technique (such as, auricular septal defect repair, pulmonary artery procedures, tricuspid and aortic valve defect repair) have been performed successfully for some time,[9,12] the beating heart technique procedure was considered an option for mitral valve replacement (MVR) surgery.
Congenital or acquired heart valve disease represents a major cardiovascular problem,[13] and the repair or replacement of diseased valves is the second most frequent cardiac operation after myocardial revascularization.[14] To our knowledge, there have been no prior studies of beating heart MVR surgery in Cuba. Therefore, we decided to conduct a study to assess the safety of beating heart MVR surgery compared with arrested heart MVR.
METHODS
Type of study and sample A randomized, controlled intervention study was conducted with a sample of 64 patients referred to the Cardiology and Cardiovascular Surgery Institute (ICCCV) in Havana for isolated MVR between January 2001 and December 2002. The research protocol was evaluated and approved by the ICCCV Ethics Committee, and patients consented to take part in the study. Sample size was consistent with recommendations in the literature consulted, which call for groups of 25 to 30 patients.[15-18] A two-year period was set for incorporating all patients who met the following inclusion criteria: a) diagnosis of mitral valve disease in any of its forms: valvular stenosis, valvular insufficiency, or double valve lesion; b) conformity with the surgical criteria for MVR;[19] c) no other associated cardiopathies, such as congenital abnormalities, significant coronary artery lesions, or other operable heart valve diseases; d) undergoing heart surgery for the first time; e) normal left ventricular systolic function (LVEF ≥ 50%); f) consent obtained.
Patients were randomly selected and evenly distributed in two surgical groups: control group A (arrested heart procedure) and study group B (beating heart procedure). Distribution of selected patients into one of the two groups was made using random numbers generated by the SIGESMU software module.[20]
The 32 patients in each group shared similar preoperative clinical profiles with the exception of sex (Table 1). Over two-thirds (68%) of patients in the study were female, a predominance consistent with the epidemiological behavior of mitral valve disease.[21] After random distribution, there were six more women than men in Group A and 18 more women than men in Group B.
Mitral Valve Replacement Surgery (MVR) All operations were performed by the same surgical team. In each patient, the diseased mitral valve was replaced with a mechanical bivalve prothesis (Bicarbon), mitral model (Sorin Biomedica Cardio, Italy). In both groups, a CPB pump (Gambro, Germany) was used.
Table 1: Preoperative Clinical Profile of Patients in Study Comparing Beating Heart and Arrested Heart Mitral Valve Replacement Surgery
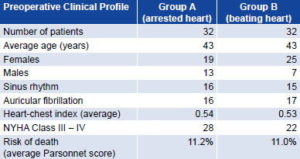
Source: Patient records. Cardiology and Cardiovascular Surgery Institute, Havana.
Control Group A (n = 32): A vascular cross clamp was placed on the ascending aorta between the arterial perfusion cannula and the cardioplegic cannula. A cold (0°C – 5°C) crystalloid cardioplegic solution was administered in proportions consistent with modified Stanford cardioplegia[2] to stop electrical and mechanical heart activity during diastole. Repeated doses of this solution were administered every 20-25 minutes to maintain cardiac arrest. To maintain perfusion under moderate hypothermic conditions, a hyper/hypothermia machine (Hemotherm, Germany) was used to lower body temperature to approximately 32°C.
Study group B (n = 32): The valve replacement procedure was carried out with continuous perfusion under normothermic conditions. A vascular cross clamp was placed on the ascending aorta between the arterial perfusion cannula and the cardioplegic cannula; however, no cardioplegic solution was administered, rather continuous perfusion of the coronary arteries was maintained with oxygenated blood from the CPB pump oxygenator through insertion of a Y-shaped line in the arterial perfusion line, which allowed the heart to continue beating during the entire operation. Body temperature was kept at 36°C – 37°C.
Variables studied Data on the following variables was collected for each patient: age, sex, heart rate, heart-chest index, New York Heart Association (NYHA) functional class,[22] estimated risk of death using the Parsonnet risk scale,[22-24] serum creatine kinase (CK) enzyme and its MB isoenzyme (CK-MB) and lactate values; duration of aortic cross-clamping, CPB, mechanical ventilation support, drainage, and postoperative bleeding; total operation time; total amount of blood loss and blood administered; length of stay in the surgical intensive care unit (SICU); length of postoperative hospital stay; and postoperative complications related to the valve prosthesis. This information was entered and stored for processing and statistical analysis in a database designed in Microsoft Office Excel for Windows.
Total CK and CK-MB were used as enzymatic markers of cellular damage and were measured before and after CPB, and on the first day after surgery. Reagents for the activated NAC ultraviolet kinetic test and the immunological activated NAC ultraviolet kinetic test (Centis Diagnostics, Cuba) were used, respectively. Total CK values of 24-170 IU (women) and 24- 190 IU (men) were considered normal. CK-MB activity values should range from 6% to 25% of total CK values. Lactate, considered a product of oxidative metabolism under anaerobic conditions, was assessed before and after CPB using the Trinder/Lactate oxidase/PAP enzymatic colorimetric method (Centis Diagnostics, Cuba). Values equal to 0.99 – 1.77 mmol/L were considered normal. To facilitate data analysis, the relative increase (R) of each variable was calculated by dividing the value obtained at each point in time (after CPB and one day after surgery) by the initial value.
The internationally accepted guidelines and recommendations proposed by the Ad Hoc Liaison Committee for defining complications related to valve prostheses, and for reporting morbidity and mortality after heart valve operations, were followed.[25,26]
Postoperative Follow-Up After discharge, all patients were referred to the cardiovascular surgery outpatient service for ambulatory follow-up care, including complete clinical evaluation and complementary tests if necessary. All patients received at least five years of follow-up before the study was closed on December 31, 2007.
Statistical analysis The following statistical tests were used: Student’s parametric t-test for comparing the mean values of quantitative variables in independent samples and the Wilcoxon- Mann-Whitney non-parametric test for comparing mean averages in independent samples when requirements for using the parametric test were not met. A confidence level of 95% was used and all p values ≤ 0.05 were considered statistically significant.
RESULTS
Values found for the serum enzyme markers of myocardial damage are shown in Table 2. Before CPB, the average values of Total CK and CK-MB were within normal parameters and were similar in both groups studied. Although none of the individuals presented clinical or electrocardiographic evidence of perisurgical myocardial infarction, the values of these enzymes doubled or tripled immediately after CPB and were three to four times higher on the first day after surgery. Differences in the increases exhibited by each group were not statistically significant (p = 0.67 for Total CK and p = 0.75 for CK-MB); however, all values were lower in Group B than in Group A. Lactate in peripheral venous blood was the same in both groups before CPB and exhibited the samebehavior as the enzymes; although the difference between the values obtained for each group after CPB was not statistically significant (p = 0.90), the increase in lactate level was less in Group B than in Group A.
Table 2: Average Increase in Serum Enzyme and Serum Lactate Variables During and After MVR Surgery
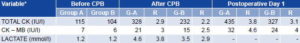
Group A (G-A): Control group, arrested heart technique
Group B (G-B): Study group, beating heart technique
CPB: Cardiopulmonary bypass
R: Relative increase = increased value divided by value before CPB
*Statistical Test: Wilcoxon-Mann-Whitney p > 0.05 (not significant)
Source: Patient records. Cardiology and Cardiovascular Surgery Institute, Havana.
Table 3 shows a similar pattern for the remaining values studied. Average aortic cross-clamping and CPB time was the same for both groups. Average surgical time, amounts of blood loss and blood administered were all slightly less in the group that underwent beating heart MVR; drainage and bleeding times were shorter for this group than for the group that underwent arrested heart MVR. Group B patients also spent less time on mechanical ventilation support, less time in the SICU, and less time in the hospital following surgery than Group A. However, none of these differences were statistically significant (p > 0.05).
Table 3: Differences in Average Surgical and Postoperative Variables Measured
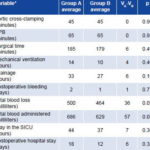
Group A: Control group, arrested heart technique
Group B: Study group, beating heart technique
VA: Average value of the variable in Group A
VB: Average value of the variable in Group B
*Statistical test: Student’s t-test p > 0.05 (not significant)
Source: Patient records. Cardiology and Cardiovascular Surgery Institute, Havana.
Although the results do not point to the superiority of one method over the other, the average values of these variables, and the differences found between the study and control groups, may be of importance from a clinical, logistical and economic standpoint. Taking mechanical ventilation support time as an example, in this study the mechanical ventilator functioned 128 hours less-almost five days-for the patients in Group B than for those in Group A. The same applies to the lower volume of blood administered to Group B, representing a savings of almost 2 liters or 4 bags of 250 ml. Similarly, total stay in the SICU was 224 hours less for the 32 patients in Group B, and the total postoperative hospital stay for this group was 192 days less than for the patients in Group A.
The differences in drainage time (192 hours less for Group B) and postoperative bleeding time (half as long in Group B) should be emphasized, considering their implications from a medical ethics standpoint, as well as the potential for reducing risk of infection.
Postoperative complications related to the valvular prostheses reported during the five-year follow-up period were similar in both groups (Table 4). Systemic thromboembolism was the most frequent complication followed by prosthetic thrombosis and hemorrhage due to anticoagulants. Incidence of prosthetic endocarditis and paravalvular leaks was minimal (only one patient with each complication) and reported only in Group A. Neither of these disorders was reported in any of the patients in Group B.
DISCUSSION
At present, mitral valve surgery requires opening the heart chambers and therefore cannot be performed without CPB;[14] it can be performed, however, with the beating heart continuous coronary perfusion technique.[27-31] Although this procedure does not prevent the harmful effects of CPB, ischemic damage to the myocardium and the reperfusion injury observed with the cardioplegic arrested heart technique can be avoided while preserving the benefits of normothermic heart surgery.[1,2]
Given that no statistically significant differences between beating heart and arrested heart MVR were observed for any of the variables assessed in this study, both myocardial protection methods can be considered equally safe, and beating heart MVR may be considered an option as effective as cardioplegic arrested heart MVR for patients with preoperative clinical characteristics similar to those of the patients who participated in this study.
Table 4: Postoperative Complications Associated with Valvular Prostheses
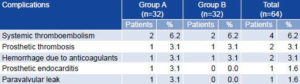
Group A: Control group, arrested heart technique
Group B: Study group, beating heart technique
Source: Patient records. Cardiology and Cardiovascular Surgery Institute, Havana.
All the patients included in the study presented low surgical risk: no other cardiopathies associated with the surgical criteria, no depressed left ventricular function (LVEF < 50%), and no prior heart surgery. It would be important to conduct similar studies with patients at greater surgical risk-for example, those needing to combine MVR with other procedures such myocardial revascularization, repair of congenital defects, or surgery on other heart valves-to determine the potential safety and benefits of beating heart MVR for those patients. In 2000, Borut et al. performed beating heart mitral valve replacement or repair in combination with other procedures in 23 patients with high surgical risk and low LVEF, using continuous retrograde coronary sinus perfusion. The authors reported good results, with only 13% early mortality; none of the deaths related to the surgical procedure. The authors noted that by preserving the threedimensional structure of the heart with this method, the surgeon could examine the mitral valve directly, before, during and after surgery, under actual physiological conditions. They therefore recommend the procedure for complex mitral valve reconstruction or even combined coronary and valvular operations in patients with high surgical risk.[32]
As noted above, the selection criteria and random distribution of patients in this study resulted in a predominance of female participants (44/64) and a greater proportion of women in both groups, with more than three times as many women as men in the beating heart group (Group B). Although this may be considered an element of bias when interpreting the results, this imbalance coincides with the epidemiological behavior of mitral valve disease, which generally affects more women than men.[21] Furthermore, the sex variable is not directly related to any of the variables used to determine the safety and benefits of each technique studied.
Both groups exhibited an increase in the cellular damage markers total CK and CK-MB following CPB and on the first day after surgery, which corroborates that damages to the myocardium and skeletal muscle always occur during on-pump MVR, regardless of the type of myocardial protection used. This coincides with findings reported by Januzzi et al. in patients without complications after heart surgery.[33] However, the small differences found in our study may suggest that beating heart MVR offers better myocardial protection, since the average values for these enzymes and for serum lactate were consistently lower in Group B, as was the relative increase obtained when comparing the postoperative increments of each variable with their average values before CPB.
These results may be considered significant from a clinical standpoint, since they suggest less severe injury or ischemic damage to the myocardium, as well as lower metabolic heart activity under anaerobic conditions, with beating heart continuous coronary perfusion MVR compared to MVR performed with the cardioplegic arrested heart technique. Other authors have reported similar findings with off-pump beating heart cardiac surgery.[5,8,34] In the case of MVR surgery, these benefits may be due to the continuous perfusion of the heart proximal to the aortic cross clamp with oxygenated blood coming directly from the CPB pump oxygenator. Blood, of course, supplies the heart with natural nutrients and greater quantities of oxygen than do crystalloid cardioplegic solutions, thus minimizing anaerobic metabolism and preventing cellular damage. Blood also supplies cells, proteins and enzymes not found in the interstitial fluid and acts as a highly effective buffer system, removing acids and increasing the oncotic activity of plasma proteins, thereby reducing interstitial edema during surgery.[1,2]
It is important to note that although total CK and CK-MB serum levels were the markers of choice for detecting myocardial damage in the past, new biochemical markers, such as myoglobin and cardiac troponins T and I, have been developed and introduced into the diagnostic toolbox. These markers are nonenzymatic components of muscle tissue that are more sensitive and cardiospecific, and capable of earlier detection of cellular damage.[33-38] However, neither these new biochemical markers nor retroplegic catheters for taking samples directly from the coronary sinus were available to us for this study; therefore, we relied on the less sensitive and less cardiospecific serum enzymes to assess myocardial and cellular damage,[34,37] and on peripheral venous serum lactate to assess the metabolic activity of the myocardium during surgery. This study was therefore limited by our inability to more accurately assess the degree of ischemic damage to the myocardium by measuring troponin and lactate in blood drawn directly from the coronary sinus. We would recommend conducting similar studies using the more specific markers and more advanced techniques.
The very slight difference in average duration of surgery-six minutes less in the beating heart group-is an interesting finding, since beating heart MVR implies operating inside a moving heart with a surgical field constantly bathed in blood. These surgical conditions suggest that the procedure should require more time, but in this study, this was not the case in practice.
Average reductions for the other variables studied point to clinical, logistical and economic advantages of beating heart MVR. For example, less mechanical ventilation time, less blood administered, less time in the SICU, and a shorter postoperative hospital stay all represent a reduction of costs during and after surgery. Patients also benefit directly from reduced drainage and postoperative bleeding time. The latter also reduces the need for blood transfusions and blood products, and consequently lowers the risks inherent in their use, which, in turn, lowers the incidence of complications, facilitating rapid postoperative recovery, which also contributes to an overall reduction in the costs associated with the procedure.[39]
The 50% reduction in postoperative bleeding time in the beating heart group compared to the arrested heart group may be explained as one of the inherent benefits of performing the procedure with normothermic perfusion, which reduces the risk of thrombocytopenia, a factor associated with increased postoperative bleeding following surgery with CPB, particularly when the cold cardioplegic technique and hypothermic CPB are used. Normothermic perfusion helps preserve the systolic and diastolic heart functions; facilitates the recovery of myocardial metabolism levels equal or very similar to pre-aortic cross-clamping levels; helps prevent intracellular calcium overload (considered the primary cause of cellular damage during myocardial reperfusion); eliminates diaphragm paralysis due to phrenic nerve injury, and thus lowers incidence of respiratory complications; reduces mechanical ventilation support time; improves spontaneous recovery of the heart’s normal sinus rhythm, and reduces the need for hemodynamic stabilization using inotropic support or an intraaortic counterpulsation balloon.[1,2]
Currently, implantation of an artificial heart valve can improve the survival and quality of life of a significant number of patients and is considered a routine treatment in cases of advanced heart valve disease.[14] However, despite continued improvement in designs, the perfect valve does not exist, and patients with heart valve prostheses are candidates for new illnesses stemming from eventual complications associated with these prostheses.
Complications that arise during the immediate postoperative period are usually related to the surgical technique applied, while those that appear later are more often the result of prosthetic dysfunction or anticoagulant treatment.[14] In this study, incidence of postoperative complications associated with valve prostheses observed in patients in both groups was similar to that reported by other authors. [13,14] In Group B, however, no cases of infectious endocarditis or paravalvular leak were reported, compared to one case each in Group A. These valvular complications are severe, often requiring re-hospitalization and re-intervention with fairly high mortality.[14] Their absence in Group B could be related to the fact that visualization of the different mitral valve structures is better with beating heart MVR and continuous coronary perfusion. Good visualization during valve replacement is essential for preserving ring-chorda tendineapapillary muscle continuity (needed to maintain good postoperative LVF) and for preventing technical errors or infectious contamination, which can cause endocarditis or paravalvular leakage.
Although no statistically significant differences were found in the variables analyzed between the two groups, the average values reported for Group B-the beating heart surgery group-were consistently more favorable from a clinical and practical standpoint. These findings should be corroborated in further studies involving larger numbers of patients.
CONCLUSIONS
Beating heart continuous coronary perfusion surgery proved to be an equally safe myocardial protection technique as the cardioplegic arrested heart procedure for MVR in patients with low surgical risk. The beating heart technique is recommended as an appropriate alternative for Cuba and for consideration in other limited-resource settings, based on its clinical benefits and possible advantages in terms of lower healthcare expenditures, which should be confirmed in future studies.
References

- González VB. Aspectos generales de la protección miocárdica en cirugía cardiaca. Arch Cardiol Mex. 2001;71(Supl 1):201-7.
- Kirklin JW, Barratt-Boyes BG. Myocardial protection during operations with cardiopulmonary bypass. In: David T, editor. Cardiac surgery. Morphology, diagnostic criteria, natural history, techniques, results and indications. 3rd ed. New York: Churchill Livingstone; 2003. p. 129-65.
- Ascione R, Talpahewa S, Rajakaruna C, Reeves BC, Lovell AT, Cohen A, et al. Splanchnic organ injury during coronary surgery with or without cardiopulmonary bypass: a randomized, controlled trial. Ann Thorac Surg. 2006;81:97-103.
- Karolak W, Hirsch G, Buth K, Legare JF. Medium-term outcomes of coronary artery bypass graft surgery on pump versus off pump: results from a randomized controlled trial. Am Heart J. 2007;153:689-95.
- Hannan EL, Wu C, Smith CR, Higgins RS, Carlson RE, Culliford AT, et al. Off-pump versus on-pump coronary artery bypass graft surgery: differences in short-term outcomes and in long-term mortality and need for subsequent revascularization. Circulation. 2007;116:1145-52.
- Tetik O, Emrecan B, Ozpak B, Yilik L, Kestelli M, Karahan N, et al. Off-pump coronary artery by-pass surgery in patients with chronic renal failure. Anadolu Kardiyol Derg. 2008;8(3):213-6.
- Herrera JM, Cuenca J, Campos V, Rodríguez F, Valle JV, Juffé A. Cirugía coronaria sin circulación extracorpórea: 5 años de experiencia. Rev Española Cardiol. 1998;51:136-40.
- Siordia ZJ, Gamez BL. Cirugía de revascularización coronaria mediante procedimientos de invasión mínima. Revisión de opciones, indicaciones y comparación con otros procedimientos. Rev Mexicana Cardiol. 2001;12(3):121-7.
- Sharony R, Grossi EA, Saunders PC. Minimally invasive aortic valve surgery in the elderly. A case-control study. Circulation. 2003;108 Suppl l:II 43-7.
- Rosengart TK, Feldman T, Borger MA, Vassiliades TA, Gillinov AM, Hoercher KJ, et al. Percutaneous and minimally invasive valve procedures. Circulation. 2008;117:1750-67.
- Pérez López H. Cirugía cardiaca con el corazón latiendo. Suplemento científico técnico del periódico Juventud Rebelde. 2000 Jul:52-3.
- Chang CH, Lin PJ, Chu JJ, Liu HP, Tsai FC, Chung YY, et al. Surgical closure of atrial septal defect. Minimally invasive cardiac surgery or median sternotomy? Surg Endosc. 1998;12:820-4.
- Akhter MW, Rahimtoola SH. Actualización clínica en valvulopatías. Rev Española Cardiol. 2007;60(4):333-41.
- Vahanian A, Baumgartner H, Bax J, Butchart E, Dion R, Filippatos G, et al. Guía de práctica clínica sobre el tratamiento de las valvulopatías. Rev Española Cardiol. 2007;60(6):625:e1-50.
- Silva Ayçaguer LC. Cultura estadística e investigación en el campo de la salud: una mirada crítica. 1ra ed. Madrid: Diaz de Santos; 1997.
- Paneque RJ. Metodología de la investigación. Elementos básicos para la investigación clínica. 1ra ed. Havana: ECIMED; 1998.
- Silva Ayçaguer LC. Diseño razonado de muestras y captación de datos para la investigación sanitaria. 1st ed. Madrid: Diaz de Santos; 2000.
- Martínez-Almagro A, Aleixandre Benavent A, Fernández Aparicio R, Ríos Díaz TJ. Terminología, método científico y estadística aplicada en ciencias de la salud. 1st ed. Madrid: Diaz de Santos; 2007.
- Gill EA, Pittenger B, Otto CM. Evaluación de la severidad y decisiones quirúrgicas en las valvulopatías. Rev Española Cardiol. 2003;56(9):900-14.
- Silva Ayçaguer, LC. Sistema General de Simulación y Selección de Muestras (SIGESMU). [CD-ROM] In: Cultura estadística e investigación en el campo de la salud: una mirada crítica. 1ra ed. Madrid: Diaz de Santos; 1997.
- Franco, S. Patología valvular mitral. In: Franco, S, editor. Enfermedad valvular cardiaca. Medellín: Colina; 2001. p. 100-32.
- Molina Méndez FJ. Estratificación del riesgo en cirugía cardiaca. Arch Cardiol Mexicana. 2002;72 Suppl. 1:S141-7.
- Granton J, Cheng D. Risk stratification models for cardiac surgery. Semin Cardiothorac Vasc Anesth. 2008;12(3):167-74.
- Hsieh CH, Peng SK, Tsai TC, Shih YR, Peng SY. Prediction for major adverse outcomes in cardiac surgery: comparison of three prediction models. J Formos Med Assoc. 2007;106(9):759-67.
- Edmunds LH, Clark RE, Cohn LH. Guidelines for reporting morbidity and mortality after cardiac valvular operations. J Thorac Cardiovasc Surg. 1988;96(3):351-3.
- Bonow RO, Carabello BA, Chatterjee K, De Leon AC, Faxon DP, Freed MD, et al. ACC/ AHA 2006 Guidelines for the management of patients with valvular heart disease. Circulation. 2006;114:e84 – e231.
- He W, Lin H, Chen M. Mitral valve replacement under beating heart in 137 cases. Zhonghua Wai Ke Za Zhi. 1996;34(11):678-80.
- Pivalizza EG, Sweeney MS. High-Dose esmolol and cardiopulmonary bypass for mitral valve replacement in the beating heart. J Cardiothorac Vasc Anesth. 1997;11(4):485-6.
- Ray VG, Gutiérrez F, Arribas JM, Puente JG, Real JG, Casinello N, et al. Cirugía mitral sin pinzar la aorta y con el corazón latiendo por toracotomía izquierda. Anal Cir Cardiaca Vasc. 2001;7(2):133-5.
- Arcas R, Glenn V, Gutiérrez F, Bautista V, Jiménez A, Arribas JM, et al. Cirugía intracardíaca. Otro enfoque del problema. Arch Cardiol Mex. 2004;74 Suppl. 2:S361-3.
- Wang J, Liu H, Xiang B, Li G, Gruwel M, Jackson M, et al. Keeping the heart empty and beating improves preservation of hypertrophied hearts for valve surgery. J Thorac Cardiovasc Surg. 2006;132(6):1314-20.
- Borut G. Mitral valve repair or replacement on the beating heart. Heart Surg Forum. 2000;3(3):232-7.
- Januzzi JL, Lewandrowski K, MacGillivray TE. A comparison of cardiac troponin T and creatine kinase-MB for patient evaluation after cardiac surgery. J Am Coll Cardiol. 2002;39(9):1518-23.
- Koh TW, Carrwhite GS, DeSouza AC. Intraoperative cardiac troponin T release and lactate metabolism during coronary artery surgery: comparison of beating heart with conventional coronary artery surgery with cardiopulmonary bypass. Heart.1999;8(5):495-500.
- Mainet D, Sorell L, Torres MB. La troponina I cardiaca: marcador bioquímico de elección del daño miocárdico. Biotecnol Apli. 2000;17:77-84.
- Quirós JJ, Villanueva H, García Barreto D. Mioglobina/ CK-MB: un método de diagnóstico rápido en el infarto agudo del miocardio. Rev Cubana Cardiol Cir Cardiovasc. 1999;13(1):40-5.
- Fontes JP, Goncalves M, Ribeiro VG. Serum markers for ischemic myocardial damage. Rev Port Cardiol. 1999;18(12):1129-36.
- Holmvang L, Jurlander B, Rasmussen C, Thiis JJ, Grande P, Clemmensen P. Use of biochemical markers of infarction for diagnosing perioperative myocardial infarction and early graft occlusion after coronary artery bypass surgery. Chest. 2002;121(1):103-11.
- Schiro G, Ruiz R, Garberi J. Reduced postoperative transfusion requirement after beating heart operations [homepage on the Internet]. Buenos Aires: Federación Argentina de Cardiología; 2001 [updated 2001 Aug 25; cited 2001 Sept 1]. Available from: http://www.fac.org.ar/scvc/ index2.htm
THE AUTHORS
Guillermo Mojena Morfa (Corresponding Author: mojena@infomed.sld.cu), general surgeon, cardiovascular surgeon. Instructor, Cardiology and Cardiovascular Surgery Institute (ICCCV), Havana, Cuba.
Julio Taín Blásquez, cardiovascular surgeon. Consulting professor, Cardiology and Cardiovascular Surgery Institute (ICCCV), Havana, Cuba.
Ángel M. Paredes Cordero, cardiovascular surgeon. Assistant Professor and Chief of Cardiovascular Surgery Services, Cardiology and Cardiovascular Surgery Institute (ICCCV), Havana, Cuba.
Horacio Pérez López, cardiovascular surgeon. Full professor and Deputy Academic Director, Cardiology and Cardiovascular Surgery Institute (ICCCV), Havana, Cuba.
José R. Llanes Echevarría, biologist. Chief of Cardiopulmonary Bypass and Perfusion Services, Cardiology and Cardiovascular Surgery Institute (ICCCV), Havana, Cuba.
Lisbeth González González, family doctor. Second-year resident in cardiology, Cardiology and Cardiovascular Surgery Institute (ICCCV), Havana, Cuba.
Submitted: July 16, 2008 Approved: October 30, 2008






