ABSTRACT
The current pandemic has rocked the lives of human beings everywhere in ways never imagined, forcing us to question where our civilization is headed. In this article, we explore and discuss scientific evidence that helps explain recent events in the context of the COVID-19 pandemic.
COVID-19 is caused by infection with a zoonotic-origin novel virus, SARS-CoV-2, that is genetically close to two coronavirus types isolated in bats. The transmission dynamics to humans from the original and intermediary hosts remain poorly understood, but it is highly likely that the SARS-CoV-2 virus infected humans after undergoing an interspecies transfer from bats to an intermediate species, and from there to human beings. Crossing the species barrier is largely fostered by industrial-scale agricultural practices that simplify original ecosystem connections by reducing biodiversity, facilitating the emergence of new infectious diseases.
The scientific community has played an exemplary role in responding to this global emergency, working to find timely, relevant solutions for governments and society as a whole. We need to take this opportunity to promote a global and open science that delves into the interrelationships of the biological, environmental, social and economic dimensions of this and other diseases while questioning current modes of production and their impact on the environment, and thus on human health worldwide.
Keywords: Coronavirus infections; communicable diseases; zoonoses; ecosystems; technology, industry, and agriculture; pandemics; global health; Mexico
INTRODUCTION
Infectious diseases were responsible for the highest burden of disease (premature deaths and disability) in the 20th century until the development of effective, affordable interventions like vaccines and antibiotics significantly reduced infection prevalence, especially in high-income countries. Thus in 1980, smallpox, which caused 500 million deaths over the past century, was declared eradicated, thanks to a strong global immunization campaign sponsored by WHO. However, without exception, infectious diseases still pose a persistent threat to human health. Nearly 10 million annual deaths worldwide (1/5 of all deaths) are linked to infectious diseases, and the overwhelming majority of these occur in low- and middle-income countries and in children under the age of 5.[1]
Lower respiratory tract infections are the main cause of death in low-income countries, and the fourth leading cause of death worldwide.[1] It is estimated that 335 infectious diseases (emerging diseases) have arisen between 1940 and 2004, 60% of which were zoonotic in origin, and 25.4% of which were caused by viral pathogens.[2] Estimates related to major outbreaks of emerging infections in the last few decades have put costs at over US$100 billion, not to mention the cost in human lives.[3]
This article’s objective is to explore and discuss scientific evidence to explain recent events in the context of the COVID-19 pandemic. We will review what is known at this writing about this emerging infectious disease, the histories of similar pandemics and epidemics, and the importance of ecological deterioration—especially that caused by agroindustry and industrial production of animal products—in the development of zoonoses. In this context, we will also discuss the vital role the scientific community has, had and should have in understanding the pathophysiological mechanisms of emerging viral diseases, deepening knowledge of their causes and developing new therapeutic options for their treatment and prevention.
CORONAVIRUS DISEASE COVID-19
COVID-19 is the disease caused by infection with a novel virus of the Coronaviridae family, SARS-CoV-2. The first cases were reported in late December 2019 in Wuhan, Hubei, China. On March 11, 2020, WHO declared COVID-19 a pandemic, and by mid-April, nearly 2 million cases had been confirmed in 185 countries, and almost 140,000 people had died from the virus.[4,5]
The SARS-CoV-2 virus is transmitted from person to person through saliva droplets and direct contact, and has an incubation period of 1 to 24 days.[6] Like other viruses, SARS-CoV-2 infects pneumocytes, possibly via the angiotensin-converting enzyme-2 (ACE2) cell receptor.[6] The first clinical manifestations of COVID-19 are fever, cough, nasal congestion and fatigue.[7] In Wuhan, 14% of cases progressed to more severe symptoms, such as dyspnea and pneumonia.[7] The estimated mortality rate is 3%,[8] although as the pandemic has progressed, this value has been continuously updated. In Mexico, the first case was recorded on February 27, 2020, and by mid-April, 5399 cases and 406 deaths had been registered.[4] Considering there is high prevalence in Mexico’s adult population of comorbidities associated with COVID-19 morbidity and mortality, particular attention should be paid not only to prevention of future outbreaks in general, but particularly to these frequently found risk factors. These include overweight/obesity, high blood pressure and diabetes mellitus (72.5%, 31.5% and 12.9%, respectively in Mexican adults).[9]
IMPORTANCE
Summarizing the SARS-CoV-2 characteristics, emergence, and transmission pathways, this article examines disruptive patterns of human activity, such as agroindustrial food production and anthropization, and their contribution to fostering recent zoonoses including COVID-19; the authors call for open-science, collaborative research that integrates biological, environmental, ecological and socioeconomic approaches to understand the root structural causes of these emerging and re-emerging zoonoses.
Using samples from infected patients in Wuhan, phylogenetic analyses of the viral genome were performed, showing that SARS-CoV-2 belongs to the subgenus Sarbecovirus of the genus Betacoronavirus, and is closely related (88% identity) to two bat coronaviruses, bat‑SL‑CoVZC45 and bat-SL-CoVZXC21. It is also related, though not as closely, to Severe Acute Respiratory Syndrome coronavirus (SARS-CoV) (±79%) and Middle Eastern Respiratory Syndrome coronavirus (MERS-CoV) (±50%). Divergence between the SARS‑CoV and SARS-CoV-2 genomes has shown SARS-CoV-2 to be a novel coronavirus whose original host was probably the bat.[5] However, it is important to note there is little likelihood that the coronaviruses bat-SL-CoVZC45 and bat-SL-CoVZXC21 are the direct ancestors of SARS‑CoV‑2. Rather than a bat, the likely intermediate host between bats and humans was being sold in the Huanan market in Wuhan, and was not hibernating during the season when the outbreak occurred.[5]
Humans had already recently experienced two epidemics with high pandemic potential, caused by two novel coronaviruses: the SARS-CoV virus (2002), which infected 8000 people and killed 774 people in 26 countries,[10] and the MERS-CoV virus (2012), which infected 2494 and killed 858.[11] Interspecies transmissions were identified in both cases, with the masked palm civet (Paguma larvata) in SARS‑CoV[12] and the camel (Camelus dromedarius) in MERS-CoV,[11] acting as intermediate hosts between human beings and the likely natural reservoir of these coronaviruses, the bat. These diseases have now been contained (not eradicated), and no vaccine or specific treatment is available for either. No SARS virus transmission has been reported in any region of the world since 2005, while some cases of MERS are reported every year, most of which are direct transmissions (host animal to human) occurring in Saudi Arabia. SARS was contained by interrupting person-to-person transmission using syndromic surveillance measures, rapid patient isolation, strict quarantine of contacts, and in some regions, quarantine of whole populations.[13]
CORONAVIRUSES AND THEIR NATURAL RESERVOIRS
Coronaviruses are RNA viruses with large genomes (26 to 32 kb), larger than that of any other RNA virus type (≤10 kb). They commonly infect birds, but also mammals, such as bats and humans, causing respiratory infections in humans and enteritis in other animals. Of the seven coronavirus subtypes known to infect human beings, the betacoronaviruses are the ones that cause severe clinical symptoms and high mortality rates.[14]
The nucleotide substitution rate in coronaviruses is ±10-4/sites per year, with mutations in each replication cycle.[15] This genomic “dynamism” has promoted origin of new viral variants capable of crossing the species barrier, adapting to a new host and achieving transmission.[16]
The bat is the natural reservoir of a wide variety of viruses, and in recent years has been shown to be the natural host of coronaviruses that are closely related to highly pathogenic betacoronaviruses, such as SARS-CoV, MERS-CoV and SARS-CoV-2.[17] Metagenomic analyses of the virome of 196 different bat species (estimates put the number of bat species worldwide at 1240) show the great variety and density of viruses, estimating that coronaviruses comprise 30% of the bat virome.[18] However, bats rarely show clinical symptoms of infection with these or other types of viruses, suggesting a history of coevolution tending toward equilibrium.
Although little studied, the immune response in bats is very similar to that in other mammals, and certain aspects are closely linked to their special relationship with the viruses. This could have a restrictive effect on certain pathogens with which they have closely evolved, preventing infection-associated immunopathology.[19] Another way of preventing immunopathological consequences of an acute immune response is through autophagy and cellular apoptosis.[19] Bats also have constitutive activation of the type I interferon system (cytosine and receptor), which regulates recruitment of macrophages and natural-killer (NK) cells to fight viral infections and tumors.[20] Lastly, some hypothesize that the increased body temperature and metabolic rate during flight may simulate a fever response that could partially explain the bats’ special tolerance to viruses.[20]
The transmission dynamics of these and other viruses (intra- and interspecies) depend on conditions associated with the virus itself, the host, the newly infected organism and the environment in which these interactions occur. In general, these conditions are: 1) frequency of interactions between the natural, intermediate and final hosts; 2) population density of the infected host species; 3) overall health status of both the host and newly infected individual; 4) specific viral characteristics and adaptations (infectiousness, pathogenicity, drug resistance, etc.); and 5) behavior of the final human host (travel, migration, conflict and war, globalization, urbanization, etc.). Changes in each of these five conditions have been behind the surge in emergence and re-emergence of viral infectious diseases, especially zoonoses, that have increased infectiousness and pathogenicity.
In the past 20 years, zoonoses such as the encephalitis outbreak associated with the Nipah virus (Malaysia, 1998), the SARS epidemic (2002), MERS (2012), Ebola (West Africa, 2014−2015) and most recently, the COVID-19 pandemic, have reminded us of the inherent, inseparable connection among all forms of life and their environment.
AGROINDUSTRIAL SYSTEMS AND THE ECOLOGY OF ZOONOSES
Current agroindustrial production systems have exerted strong pressure to change land use in extensive areas of the temperate and tropical regions, creating a continuum of heavily anthropized agroecosystems that increasingly encroach on less suitable land, which then undergoes considerable habitat deterioration and a major loss of ecosystem services that these spaces provide.[21] Especially the tropics, the habitat of a highly diverse population of birds and bats, have been deforested in the last few decades for the industrial exploitation of palm oil, rice, soy or sorghum for livestock feed, or for the planting of forage grasses to create pastures and farms for intensive ruminant, pig and poultry production.[22] Habitat loss in these highly biodiverse ecosystems reduces the original wild populations, creating imbalances in all trophic relationships, including changes in endosymbiosis and ectosymbiosis due to the physiological stress associated with the drastic change from original ecological conditions.
The surviving species are forced to explore new ecological niches, interact with species with which they had no prior contact, modify their geographic distribution beyond the optimal bounds for their physiology, and adjust their interactions with a virome also in the process of modification through contact with new vectors and potential hosts.[23]
In general terms, when an ecosystem undergoes agricultural anthropization, it remains in a perpetual state of disruption. This favors less specialized species that are highly adaptable to the dynamics of rapid growth cycles with a high input of nutrients and xenobiotics, frequent disturbances from machinery and infrastructure abuse, and overrepresentation of a few species over broad areas and time periods. In other words, the ecosystem has been artificially blocked from maintaining or reaching its natural equilibrium. It constantly recruits the individuals most resistant to the pressures exerted by agricultural management on an industrial scale, which includes the pressures exerted by pathogens favored in the continuum of degraded ecosystems. For birds and bats, the new wooded areas in plantations and paddocks, as well as islands of original vegetation, become sites of intense competition between the species most favored by constant disruption. This competition can establish new viral exchanges and recombination dynamics among the survivors and livestock.[22] Major selective pressure is also exerted by the introduction of agrochemicals and bio-inputs, especially on arthropod populations, and consequently on the bats and birds associated with them on the trophic level. These bats and birds, along with some rodents, may form part of the most overrepresented wild populations in the ecosystem, and are candidates for becoming new viral reservoirs.[24]
In this context, it is understandable that the accelerated rates of generational succession artificially imposed by agroindustry also favor the selection of new strains of microorganisms and viruses capable of crossing the species barrier, including the jump to humans, with varying degrees of pathogenicity. Migration of wild fauna, whether habitual or forced, constantly alters the distribution map of many viruses, moving these into new areas, and changes their choice of host and vector species. At the same time, worldwide movement of livestock production (live animals) with human populations guarantees redistribution of these viruses into the continuum of anthropized ecosystems, despite health controls.
Other ecological aspects of certain bird and bat species—such as the plasticity of their reproductive rates, gregarious and itinerant habits, greater tolerance of nearby human populations, and possibly the rapid elimination of those individuals that develop acute reactions to viruses—have identified them as key participants in most emerging infectious disease outbreaks in the last few decades.[16] In some cases, the disturbed ecosystem continuum favors these species, due to an incomparable food supply and the relative absence of predators and competitors, which allow their populations to grow beyond the limits imposed by a more biodiverse ecosystem.[25] This promotes the growth of virus populations and their concomitant evolution, and favors the emergence of lineages capable of progressing in novel hosts, including humans.
It should also be noted that agroindustrial operations are financially successful because of the high genetic homogeneity of farmed species, permitting standardization of processes, supplies, machinery, facilities and products to constantly meet high market demand. Thus, large numbers of animals of the same age, sex and genetic vulnerability are confined in small spaces, with overly enriched diets and high chronic stress levels that guarantee success for infections and ample opportunities for new mutations. For example, of the 41 reconversion events in highly pathogenic avian influenza viral strains reported from 1959 to 2015, specifically subtypes H5 and H7, only two occurred on backyard farms, while the rest were identified in industrial scale commercial operations. However, even the two small-farm events took place in areas where there was industrial-scale poultry farming.[26]
Thus, it is essential to investigate the extent to which industrial modes of food production, especially those of animal origin, favors the emergence of recent zoonoses. Figure 1 illustrates the interrelation among the biological, environmental, ecological and socioeconomic factors of emerging zoonoses, pointing the need for new research approaches that integrate each of these dimensions when addressing these types of health challenges.
The high human and economic costs of these emerging diseases must no longer be ignored. It is time for us learn our lesson and stop placing the financial interests of a few corporations or states that control large-scale animal production ahead of global health, since it is clear that there is only “One Health.” We must restore ecosystems that have suffered profound destruction under current models of industrial agriculture and global commerce (including both their supplies and products). An open, global science must delve into the root structural causes of emerging viral diseases that are crossing species barriers and threatening humanity.
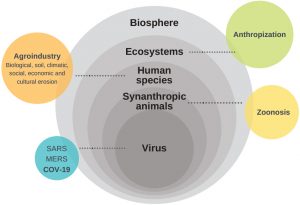
Figure 1: Interrelation among biologic, environmental, ecologic and socioeconomic factors in emerging zoonoses
THE NEED FOR CRITICAL, THOUGHTFUL AND SOCIALLY-COMMITTED SCIENCE
From the first alarm sounded by Chinese health authorities and WHO’s declaration of COVID-19 as an international emergency, the scientific community has focused on generating useful information, mainly regarding the epidemiology of the disease, initial evidence of the natural history of the virus, clinical characteristics of the disease and measures for its control and treatment. This time, the information generated has been openly distributed. From December 1, 2019 until mid-April 2020, 2832 scientific studies on COVID-19 have been published in international journals indexed in English.[27] Some of the studies discussed in this article are examples of this intense effort of solidarity by the scientific community.
Publication of initial observations enabled exchange of knowledge and experiences, facilitating a global response to the pandemic through open access and coordinated actions. Interestingly, the first articles published on COVID-19 in The Lancet (in all its various formats and special editions) were quickly translated into Mandarin for dissemination, mainly among health professionals in China.[28] The scientific community has also played an essential role in releasing technical information to the general public (feature articles, infographics, interactive maps, etc.), which has aided dissemination of useful information for understanding the outbreak and its transmission, as well as the reasoning behind measures taken for its containment and their implementation to prevent contagion.
Participation by global organizations such as WHO has been vital to foster international cooperation and mobilize coordinated actions to confront the pandemic. At the beginning of February, 2020, WHO organized a virtual forum of scientists from the world over to establish research priorities for COVID-19.[29] Nine research priorities were proposed for medium- and long-term control of the pandemic; among these were topics on the virus’s natural history (origin, transmission dynamic and measures to manage it) and development of vaccines and therapies to prevent and treat the disease. WHO also has developed a public registry of clinical trials being carried out worldwide; by mid-April, this platform already contained 1135 records of clinical trials evaluating COVID-19 interventions.[30]
At the start of the outbreak in China, Mexico’s National Council of Science and Technology (CONACYT) created a National Project on COVID-19 Research and Social Impact (PRONAII COVID-19) as part of the National Strategic Health Program (Pronaces-Health). The latter is a high-priority initiative for organizing research efforts on important national issues that call for a decisive focus and an integrated, in-depth, broad-ranging solution. To date, CONACYT and the Mexican scientific community are working on 14 Pronaces initiatives, including Pronaces on Health, a funding opportunity for integrated multidisciplinary long-term research projects aimed at providing evidence for actions to solve strategic challenges for promoting health in Mexico.
Led by the Mexican scientific community in coordination with the Federal Health Ministry, PRONAII COVID-19 has identified urgent challenges and capacities, and projects were developed to give immediate attention to the emergency. These included projects concerning epidemiological surveillance, mathematical modeling and data science, clinical trials, vaccine development, and creation of sensitive and specific detection tools, as well as the search for means to manufacture mechanical ventilators in Mexico.
The second phase of the strategy thus far has been to publish two specific calls for proposals: one for cutting-edge research in all relevant fields, including health technology development and innovation, and the other to strengthen Mexico’s ability to equip laboratories at academic institutions to provide COVID-19 diagnostic support. Certainly, thanks to the proactive efforts of researchers at several public research centers, CONACYT was able to drive immediate actions, including manufacture of strategic medical devices such as mechanical ventilators and industrial production of hydroalcoholic gel to supply public hospitals.
These efforts clearly exemplify the relevance of intersectoral work and the fundamental role of an ever more conscious, critical and participatory scientific community to meet and overcome challenges like the COVID-19 pandemic, with a collaborative rather than competitive approach. Simply put, the scientific community’s response to COVID-19 is evidence of the political, economic and scientific strengths of Mexico, when committed to clear goals aimed at improving the population’s health and well-being.
PRIORITIES AND PROSPECTS FOR FUTURE RESEARCH
Effective tools for containment (improvements in health systems), treatment (new, more sophisticated drugs) and prevention (vaccines and more sensitive diagnostic devices) have been developed for most infectious diseases over the past 50 years. Yet, in just the last 20 years, the SARS, MERS, Ebola and COVID-19 outbreaks have demonstrated the need for greater, more collaborative and more diversified efforts to identify sustainable solutions to current and future challenges.
Funding for scientific research in the field of infectious diseases should consider the burden of these diseases at the regional and global level and the fact that they are the fourth leading cause of death worldwide.[1] Given that other threats such as the COVID‑19 pandemic may be imminent, it is also crucial to strengthen each sector’s capacity for response. For example, development of vaccines and biologics is critical and requires constant impetus. After the SARS outbreak in 2002, several studies focused on development of anti-SARS-CoV neutralizing antibodies, but none of the candidates has yet been evaluated in clinical trials,[31] and thus could not be immediately used to evaluate efficacy for the new virus (they are cross-reactive against SARS-CoV-2).
It is important to learn from this and prior experiences. In addition to constantly analyzing and keeping records of observations and research during this type of challenge, we consider it essential to encourage collaborative discussion and reflection from multidisciplinary perspectives, which will aid in understanding the root structural causes of these new viral diseases, in addition to providing solutions to the challenges they present.
Public release of technical information and scientific research dissemination are the best tools to enable both civil society and authorities to make informed, accurate decisions. Truth is both an ethical and technical imperative as humanity confronts challenges like that presented by COVID-19, which is one reason why the scientific community’s participation is vital. In such cases, it is capable of anticipating, exploring and understanding in depth the ultimate and structural causes of these diseases as well as the challenges involved in their containment, mitigation, treatment and prevention, providing relevant and logical solutions for governments and society at large.
During this global health emergency, we are also challenged to seek more democratic, open ways to share scientific knowledge, to avoid subordination of epistemological principles and the best solutions or prevention to the financial interests of the big pharmaceutical corporations. We must be innovative in the ways science supports decision-making and also build bridges of dialogue and collaboration among sectors and countries, between the Global North and the Global South, and more. It is also urgent to establish mechanisms to prevent the spread of misinformation and the abuse of social networks to this end.
International collaboration has been our core strength in confronting the current COVID-19 pandemic, as in past pandemics and epidemics. The interconnectivity of the world in which we live is not only virtual, but physical; and it is not only among humans, but also with the ecosystem. The “One Health” concept, institutionalized by WHO since 2008, encompasses these ideas. Its strategy proposes using a systemic, interdisciplinary and multisector approach to design and implement programs, policies, legislation and research to improve the health of all populations in the ecosystem—and of the ecosystem itself—at local, regional and global levels. At the same time, this concept calls for a deeper questioning of modes of production and their impact on the environment, an environment also shared on a global level and inseparable from human health.
References

- GBD 2016 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017 Sep 16;390(10100):1260–344.
- Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, et al. Global trends in emerging infectious diseases. Nature. 2008 Feb 21;451(7181):990–3.
- United Nations Environmental Programme. UNEP Frontiers 2016 Report. Emerging Issues of Environmental Concern [Internet]. Nairobi: United Nations Environmental Programme; 2016 [cited 2020 Apr 11]. 73 p. Available at: http://wedocs.unep.org/handle/20.500.11822/7664
- Johns Hopkins University [Internet]. Baltimore: Johns Hopkins University; c2020. Johns Hopkins Coronavirus Resource Center; [cited 2020 Apr 11]. Available at: https://coronavirus.jhu.edu/map.html
- Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020 Feb 22;395(10224):565–74.
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020 Mar;579(7798):270–3.
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of Coronavirus disease 2019 in China. N Engl J Med. 2020 Feb 28. DOI: 10.1056/NEJMoa2002032. [Epub ahead of print]
- Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 Feb 24. DOI: 10.1016/S2213-2600(20)30079-5. [Epub ahead of print]
- Encuesta Nacional de Salud y Nutrición (ENSANUT) 2018 [Internet]. Aguascalientes (MX): INEGI; National Institute of Health (MX); 2018 [cited 2020 Apr 19]; [2.13 MB]. Available at: https://www.inegi.org.mx/programas/ensanut/2018/. Spanish.
- Peiris JSM, Guan Y, Yuen KY. Severe acute respiratory syndrome. Nat Med. 2004 Nov 30;10(12 Suppl):S88–S97.
Al-Tawfiq JA, Memish ZA. Middle East respiratory syndrome coronavirus: Transmission and phylogenetic evolution. Trends Microbiol. 2014 Oct 22;22(10):573–9.
- Guan YJ, Zheng BJ, He YQ, Liu XL, Zhuang ZX, Cheung CL, et al. Isolation and characterization of viruses related to the SARS coronavirus from animals in Southern China. Science. 2003 Oct 10;302(5643):276–8.
- Wilder-Smith A, Chiew CJ, Lee VJ. Can we contain the COVID-19 outbreak with the same measures as for SARS? Lancet Infect Dis. 2020 Mar 5. DOI: 10.1016/S1473-3099(20)30129-8. [Epub ahead of print]
- Su S, Wong G, Shi W, Liu J, Lai ACK, Zhou J, et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016 Jun;24(6):490–502.
- Pyrc K, Dijkman R, Deng L, Jebbink MF, Ross HA, Berkhout B, et al. Mosaic structure of human Coronavirus NL63, one thousand years of etyvolution. J Mol Biol. 2006 Dec 15;364(5):964–73.
- Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019 Mar;17(3):181–92.
- Li W, Shi Z, Yu M, Ren W, Smith C, Epstein JH, et al. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005 Oct 28;310(5748):676–9.
- Chen L, Liu B, Yang J, Jin Q. DBatVir: The database of bat-associated viruses. Database. 2014;2014:1–7.
- Brook CE, Dobson AP. Bats as “special” reservoirs for emerging zoonotic pathogens. Trends Microbiol. 2015 Mar 1;23(3):172–80.
- O’Shea TJ, Cryan PM, Cunningham AA, Fooks AR, Hayman DT, Luis AD, et al. Bat flight and zoonotic viruses. Emerg Infect Dis. 2014 May;20(5):741–5.
- Cascio A, Bosilkovski M, Rodríguez-Morales AJ, Pappas G. The socio-ecology of zoonotic infections. Clin Microbiol Infect. 2011;17(3):336–42.
- Afelt A, Frutos R, Devaux C. Bats, coronaviruses, and deforestation: toward the emergence of novel infectious diseases? Front Microbiol. 2018 Apr 11;9(Art 702):1–5.
- Plowright RK, Eby P, Hudson PJ, Smith IL, Westcott D, Bryden WL, et al. Ecological dynamics of emerging bat virus spillover. Proc Biol Sci. 2015 Jan 7;282(1798):20142124.
- Allen T, Murray KA, Zambrana-Torrelio C, Morse SS, Rondinini C, Di Marco M, et al. Global hotspots and correlates of emerging zoonotic diseases. Nat Commun. 2017 Oct 24;8:1124.
- Chan JFW, Kai-Wang to K, Tse H, Jin DY, Yuen KY. Interspecies transmission and emergence of novel viruses: lessons from bats and birds. Trends Microbiol. 2013 Jun;21(10):544–55.
- Dhingra MS, Artois J, Dellicour S, Lemey P, Dauphin G, Von Dobschuetz S, et al. Geographical and historical patterns in the emergences of novel highly pathogenic avian influenza (HPAI) H5 and H7 viruses in poultry. Front Vet Sci. 2018 Jun 5;5(Art. 84). DOI: 10.3389/fvets.2018.00084.
- PubMed. Home – PubMed – NCBI [Internet]. Maryland: National Institutes of Health; c2020 [cited 2020 Apr 14]. Available at: https://www.ncbi.nlm.nih.gov/pubmed
- Xiang YT, Li W, Zhang Q, JinY, Rao WW, Zeng LN, et al. Timely research papers about COVID-19 in China. Lancet. 2020 Feb 29;395(10225):684–5.
- World Health Organization. A Coordinated Global Research Roadmap: 2019 Novel Coronavirus. Geneva: World Health Organization; 2020 Mar. 36 p.
- World Health Organization [Internet]. Geneva: World Health Organization; c2020. International Clinical Trials Registry Platform (ICTRP).Welcome to the WHO ICTRP; 2020 [cited 2020 Apr 14]. Available at: https://www.who.int/ictrp/en/
- Jiang S, Hillyer C, Du L. Neutralizing antibodies against SARS-CoV-2 and other human Coronaviruses. Trends Immunol. 2020 Apr 24. DOI: https://doi.org/10.1016/j.it.2020.03.007.
THE AUTHORS
Mariana Cárdenas-González MS PhD, chemist with a master’s degree in environmental sciences and a doctorate in toxicology. National Council of Science and Technology (CONACYT), Mexico City, Mexico.
Elena R. Álvarez-Buylla MS PhD (Corresponding author: elenabuylla@protonmail.com), biologist with a doctorate in botanical sciences. National Council of Science and Technology (CONACYT), Mexico City, Mexico; Department of Functional Ecology, Institute of Ecology, National Autonomous University of Mexico; Center for Sciences of Complexity, National Autonomous University of Mexico, Mexico City, Mexico.
Submitted: April 20, 2020 Approved: April 28, 2020 Disclosures: None




