ABSTRACT
INTRODUCTION Human papillomaviruses and Chlamydia trachomatis are the most frequent causes of sexually transmitted infections. Although the association between some human papillomavirus genotypes and cervical cancer has been demonstrated and Chlamydia trachomatis infection is the most common cause of female infertility, Cuba has no national baseline studies on the circulation and co-circulation of these agents, the synergistic effect of which may be a risk factor for occurrence and development of precancerous cervical lesions. Additionally, few local studies have examined risk factors for infection.
OBJECTIVE Determine the frequency of infection by human papillomavirus and Chlamydia trachomatis and their association with sociodemographic, clinical and epidemiological variables in women seeking routine Pap smears or other medical services at the primary care level in Cuba.
METHODS A cross-sectional study was conducted among 500 women aged 16–67 years (100 from Havana, 200 from Villa Clara and 200 from Holguín Provinces, Cuba), from August through December 2015. Chlamydia trachomatis infection was detected by real-time polymerase chain reaction and 35 genotypes of human papillomavirus by low-density microarray. We then examined the association of infection with sociodemographic, clinical and epidemiological variables.
RESULTS Human papillomavirus was detected in 14.8% (74/500) of the women. Of the 29 genotypes identified, 79.7% (59/74) were oncogenic high-risk types. Type 16 was the most frequently identified (23%; 17/74), followed by type 31 (10.8%; 8/74) and then by types 33, 53, 61 and 66 in equal proportions (8.1%; 6/74). Infection frequency was greater in women aged ≤25 years (38.8%; 31/80), students (46.7% 7/15), single women (23.0%; 40/174) and among those who reported having more than 3 sexual partners in the last 2 years (41.5%; 17/41). Differences were found among provinces for circulating genotypes and infection-related variables. Human papillomavirus infection from genotypes 16, 31, 33, 53, 61, 66, 68 and 89 was associated with the 7.9% (30/382) of women who had positive Pap tests. Infection from Chlamydia trachomatis was positive in 1% (5/500) of women, all aged ≤25 years. Coinfection by Chlamydia trachomatis and HPV was found in one woman infected with human papillomavirus genotype 61.
CONCLUSIONS Frequency of human papillomavirus is high in the three Cuban provinces studied, with greater frequency of genotype 16 and other oncogenic high-risk types. For both agents, infection is more frequent in young women and adolescents. Positive Pap tests are frequently associated with HPV infection. Prevalence findings from this study could be used as a baseline for future research or interventions.
KEYWORDS Human papillomavirus, genotypes, Chlamydia trachomatis, neoplasms, sexually transmitted diseases, cervix Uteri, infection, real-time polymerase chain reaction, women, Cuba
INTRODUCTION
Human papillomavirus (HPV) and Chlamydia trachomatis are among the most frequent causes of sexually transmitted infections (STI) in the world. WHO estimates that close to 290 million women have HPV, and approximately 131 million chlamydia cases are reported annually. The latter is the most frequent bacterial STI. Despite advances in STI diagnosis, treatment and prevention, these two infections still constitute a global health problem.[1]
HPV infection is a necessary but not sufficient condition for appearance and development of cervical cancer, which is the main cause of anogenital neoplasms.[2] Cervical cancer ranks fourth in cancer incidence in the world.[3] Close to 200 HPV genotypes have been described, and approximately 15 to 19 are high-risk HPV based on their oncogenic potential.[4] HPV types 16 and 18 are the high-risk HPV genotypes most frequently associated with precancerous lesions and cervical cancer.[5]
IMPORTANCE This paper characterizes frequency of HPV and Chlamydia trachomatis infection in women of three Cuban provinces and highlights the need to study chlamydia infection in young and adolescent women. It supports the need to include HPV vaccination in the National Immunization Program and contributes knowledge about HPV genotypes in Cuba potentially useful for considering vaccine candidates for domestic production.
Since 1968, Cuba has implemented a cervical cancer early diagnosis program,[6] now carried out through the national network of neighborhood family doctor-and-nurse offices. The program screens sexually active women aged 25 to 59 years, for whom protocols indicate a Pap test every 3 years. The program may have contributed to the decline in cervical cancer mortality rates from above 20 per 100,000 women in 1965 to 7.7 per 100,000 in 2010. However, since then, this rate has increased. In 2016, it reached 9.1 per 100,000 and in 2018, 9.7 per 100,000. Although these rates are among the lowest in Latin America, the increases are cause for concern.[7]
Countries report different HPV prevalences by age group: in some, infection is concentrated in women aged <24 or <35 years, while in others, prevalence is also high in older women.[8] Several studies in Cuba have included populations considered at risk due to positive Pap tests or age >30 years with a positive or negative Pap. Prevalence was 94.3% in women with a positive Pap, and 41.4% in those with a negative Pap, with high frequency of oncogenic genotypes (>80%), particularly HPV 16, 18, 31, 45, 52 and 58. The factors most commonly associated with infection were low educational level, smoking, alcohol use and perimenopausal status. The factors associated with precancerous cervical lesions in these HPV-infected women were oral contraceptive use for over five years and a history of positive Pap tests or colposcopies.[9,10]
Despite these findings and the known relationship between HPV and cervical cancer, at the time of this writing, no national baseline studies have been conducted on HPV prevalence and circulating genotypes in Cuba’s female population. Due to shortcomings in prior research, limited to risk groups in a single city, it is of interest to learn frequency of HPV circulation and behavior of sociodemographic and epidemiological variables associated with infection in open populations in several regions of the country.
Most chlamydia infections are asymptomatic and frequently are neither diagnosed nor treated, leading to persistent infections that can cause pelvic inflammation, ectopic pregnancy and tubal factor infertility.[11] Infertility in Cuba is estimated at 12%–14% of couples, in which 40%–50% of causes are associated with the female partner. Tubal defects are one of the most frequent causes of female infertility,[12–14] making this infection an important health problem in Cuba.
For the past several years, the Cuban public health system has been using rapid chlamydia tests for diagnosis. However, due to the their lack of specificity, false positives occur from cross-reactions with antigens from other micro-organisms, and frequencies of up to 80% have been reported in women with lower abdominal pain.[15] Nucleic acid amplification testing has greater sensitivity and specificity for chlamydia diagnosis, but the cost is prohibitive for broad use in clinical diagnosis in low- and middle-income countries. In Cuba, these tests have been used in a few studies to estimate frequency of chlamydia infections, either by use of polymerase chain reaction (PCR) or real-time PCR. A small number are also reserved for patients with a history of infertility, those who are HIV positive, or who have symptoms of a genitourinary system disease in which prevalence fluctuates from 1% to 6%.[15–17] Thus, only a few Havana hospitals use these methods, which is why there is no data on chlamydia circulation in open populations.
The objective of this study was to determine the frequency of HPV and chlamydia infections in sexually active Cuban women with no genitourinary symptoms who sought routine services at the primary care level, as well as co-circulation of both agents and their association with relevant sociodemographic, clinical and epidemiological variables.
METHODS
Design and subjects A cross-sectional study was conducted from August through December 2015 of 500 women in 3 Cuban provinces: 100 in Havana (western Cuba), 200 in Villa Clara (central) and 200 in Holguín (eastern), with ages ranging from 16 to 67 years (mean: 38; median: 40). The provincial capital cities were chosen to include representation from the country’s three geographical regions (west, central and east) and to identify possible differences among them.
Women were included who sought primary care services: 382 for the routine Pap test included in the cervical cancer screening program and 118 outside the program’s age range of 25–59 years who sought services for other reasons, were sexually active, and who expressed interest in being tested for HPV and chlamydia.
Women were excluded if they were pregnant, HIV positive or if they had been diagnosed with any cervical disease.
After agreeing to participate by providing written informed consent, participants completed a questionnaire containing the study variables: age, educational level, occupational status, marital status, smoking status, parity and number of sexual partners in the past two years, age of onset of sexual activity, history of STIs, regular condom use, and use of intrauterine devices (IUDs) and oral contraceptives.
Two age groups were established: ≤25 and >25 years. Educational level was stratified according to Cuba’s national education system: primary, secondary, high school or vocational school, and university. Marital status was classified as either single or married/with stable partner. Age of onset of sexual activity was divided into three categories: ≤15 years, 16–20 years and >20 years, and number of sexual partners was categorized as 0, 1, 2 and ≥3.
Pap tests were done on 382 women. Those aged <25 years (80) or >59 years (38) were excluded because they were outside the age range of the national cervical cancer screening program.
Sampling Cervical cells were obtained for the Pap test.[18] Following this, an endocervical brush (Digene Inc., USA) was inserted into the endocervical canal and after extracting it, brushed around the exocervical region to obtain endo- and exo-cervical cells and extract DNA. Cells were kept in a preservation solution (Digene Inc., USA) and stored at -20 oC until processing by the virology department’s STI Laboratory at the Pedro Kourí Tropical Medicine Institute, where DNA extraction and HPV and chlamydia detection were carried out.
DNA extraction Cell suspensions were homogenized through vigorous vortex mixing (Vortex Mixer VM-10, DAIHAN Scientific Co., Ltd., South Korea) and 200 µL were taken to extract DNA using the commercial QIAamp DNA Mini Kit (QIAGEN, Germany), following manufacturer’s instructions. From 50 to 100 ng/mL of DNA was obtained from the 200 µL of homogenized cervical cell suspension. DNA was diluted in 100 µL of elution buffer and stored at -20 ºC until detection of HPV and chlamydia were carried out.
HPV detection and genotyping Using low-density microarrays, HPV detection and determination of genotypes was done with the commercial CLART HPV 2 kit (Genómica, Spain), following manufacturer’s instructions. A 450-base pair fragment in the virus’s L1 region was amplified via PCR (AERIS BG096, ESCO Micro Pte., Ltd., Singapore) and hybridization was done with specific probes for each viral genotype. Ten µL of purified DNA from each clinical sample were used in the amplification reactions. Each microarray included an internal DNA control from the clinical sample, an external amplification control and a control for marking and visualization of the amplified products. Analytic sensitivity of the assay was calibrated by the manufacturer using commercial plasmids cloned from each viral genotype. Probes were used to identify the 35 HPV genotypes of greatest clinical importance: low-risk HPV (6, 11, 40, 42, 43, 44, 54, 61, 62, 71, 72, 81, 83, 84, 85 and 89) and high-risk HPV (16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 70, 73 and 82). High or low risk of genotypes was established in accordance with the criteria of the International Agency for Research on Cancer (IARC).[19]
Chlamydia trachomatis detection using real-time PCR Chlamydia detection was done with real-time PCR at the Pedro Kourí Tropical Medicine Institute (IPK), following the Wei protocol.[20] A pair of primers that amplify a preserved fragment within the tryptophan synthetase gene of the chlamydia genome and a TaqMan (ThermoFisher Scientific, USA) probe were used. Ten µL of purified DNA were taken from each clinical sample and from the positive control, which consisted of a Chlamydia trachomatis reference strain, LGV Serovar II (ATCC VR-902BD). Negative controls consisted of 10µL of molecular-biology–grade sterile water and 10 µL of human fibroblast DNA (ATCC, CCL 171).
Data collection and handling During sampling, personal and epidemiological data were also collected, through a questionnaire designed for this purpose. Each patient’s laboratory test results were added, and all information was stored in an Excel database. Each patient was assigned a unique identification number to protect confidentiality.
Statistical analysis A database for variable analysis was developed in the SPSS statistical package version 19.0 (IBM Inc., USA). Absolute and relative frequencies of HPV and chlamydia were calculated, along with the specific HPV genotypes detected and the multiple infections diagnosed in study participants.
Ethics The project was approved by IPK’s Research Ethics Committee. Study participants provided written informed consent per the Declaration of Helsinki[21] and guidelines of the Council for International Organizations of Medical Sciences.[22] The signed document contained the necessary information about the research study, including possible benefits and risks. Participants were informed that they could withdraw from the study at any time without penalty or any effect on their care and treatment. Data were saved following the principle of confidentiality and individual identities were not revealed. Sampling was done by a trained clinician, taking the necessary care to minimize risks in accordance with good clinical practice standards. Laboratory methods were chosen according to principles of maximum beneficence and non-maleficence according to good laboratory practice standards.
 RESULTS
RESULTS
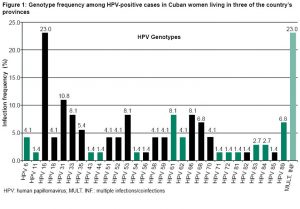
Of the 500 women examined, 14.8% (74/500) were infected by one or more HPV genotypes. A total of 29 genotypes were identified, of which 79.7% (59/74) were high-risk HPV. Genotype 16 was the most frequent, followed by 31, and then 33, 53, 61 and 66, in equal proportions (Figure 1). Low-risk HPV genotypes were found in 27% (20/74) of positive cases, with coinfection with high-risk HPV in 6.7% (5/74) Coinfections with several HPV genotypes were identified in 3.4% (17/500) of all women in the sample and in 23% (17/74) of infected women (Figure 1).
Women with multiple infections were positive for at least one high-risk HPV type. Among coinfected women, 58.8% (10/17) had 2 genotypes, 35.3% (6/17) 3 genotypes, and 5.9% (1/17) >3 HPV genotypes. No particular combinations were seen in these infections, although genotypes 16, 68, 53 and 31 were the most frequent in the coinfections identified: 35.3% (6/17); 29.4% (5/17); 23.5% (4/17), and 23.5% (4/17).
Small differences were found among provinces concerning HPV infection prevalence: Havana, 18% (18/100); Holguín 15% (30/200); and Villa Clara 13% (26/200).
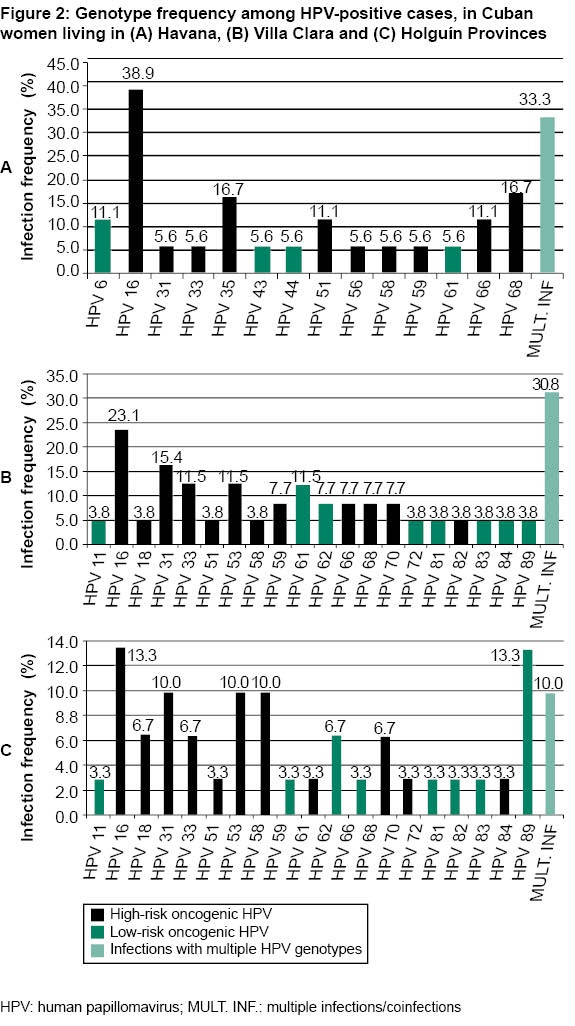
Figure 2 shows frequencies of different genotypes by province. In Havana residents, 14 genotypes were identified and, of them, 10 (71.4%) were high-risk HPV. The proportion of women infected by HPV 16 was greater in Havana (Figure 2A). For high-risk HPV 35 and 68, differences were also found between Havana and the other provinces. These genotypes were detected in equal proportion in Havana residents (Figure 2A).
In Villa Clara Province, 20 HPV genotypes were identified, of which 12 (60%) were high-risk HPV. Figure 2B shows frequency of diagnosis for the different genotypes in this province. Prevalence of HPV 31 and 33 were greater in women in Villa Clara compared with the other provinces studied.
In Holguín Province, 19 viral genotypes were detected, of which 11 (57.9%) were high-risk HPV. Frequencies of the different genotypes in this province are shown in Figure 2C. Genotype 16 was less frequent in women in Holguín than those in Havana and Villa Clara.
HPV coinfections in women were more frequent in Havana than in Villa Clara and Holguín (Figure 2). In comparing coinfection frequencies among provinces, differences were found between women residing in Havana and Holguín: 6/18 (33.3%) vs. 3/30 (10%).
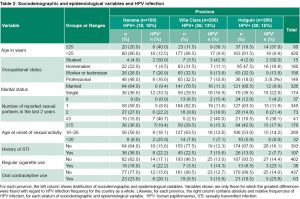
Table 1 shows infection frequencies by sociodemographic variables. Note that infection was almost 4 times greater in women aged ≤25 years than in women over this age. The proportion of infected women with only a primary-level education was twice that of university-educated women. Greater frequencies are concentrated in students, single women, smokers and in oral contraceptive users. The percentages of those who tested positive increased as the number of sexual partners in the last two years increased and the earlier women started having sexual relations. Although women with a history of STIs had the greatest frequency, there were only small differences between them and those with no STI history. We also found only a small difference related to IUD use, but women who reported regular condom use had slightly greater frequencies than women who did not (Table 1).
HPV-positive frequencies differed little among the provinces, with 18%, 13% and 15% in Havana, Villa Clara and Holguín, respectively. Infection frequencies according to sociodemographic variables by province are shown in Table 2 and generally reproduce the countrywide pattern for variables that represent the greatest risk of infection.
The data indicate differences in sociodemographic patterns by province, and show that, especially for women in Havana, the variables most closely associated with infection are single status, a high number of sexual partners, history of STIs, early onset of sexual activity, smoking and use of oral contraceptives (Table 2).
Coinfections with several HPV genotypes were more frequent in women aged 15 to 25 years and were detected in 10% (8/80) of positive cases and in 6.3% (11/174) of single women. No relationship was found between presence of coinfections and other variables associated with higher risk of infection.
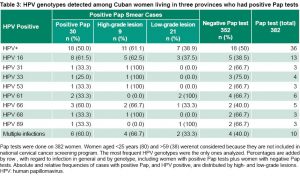
Cervical lesions were detected in 7.9% of the women who received a Pap test (30/382) and HPV infection was found in a high percentage of positive Pap smears (60%, 18/30). For intraepithelial lesions, 30% (9/30) had high-grade lesions and 70% (21/30) had low-grade lesions. Table 3 shows the association between lesions of both grades with high- and low-risk genotypes. High-risk HPV 16 and 66 were more frequently associated with positive Pap tests. HPV 16 was the most frequent in both types of lesions.
Among high-risk genotypes, genotypes 16 and 66 were the most frequent in high-grade lesions, and low-risk genotypes 61 and 89 were identified in greater proportions in low-grade lesions (Table 3).
Chlamydia was diagnosed in 1% (5/500) of women studied. All (100%, 5/5) were aged ≤25 years, and represented 6.3% (5/80) of that age group. Of these five women, two lived in Havana, two in Holguín and one in Villa Clara.
Eighty percent (4/5) of those infected had a secondary educational level (secondary, high school or vocational), 60% (3/5) were single and 60% (3/5) were homemakers. No association was found between chlamydia infection and these sociodemographic variables. Presence of chlamydia was associated exclusively with age (age ≤25 years). Coinfection with chlamydia and HPV was found in one (20%) woman infected with genotype 61.
 DISCUSSION
DISCUSSION
Anogenital HPV is the most frequent STI in the world, given that close to 90% of the sexually active population has the virus.[23] The infection is common in adolescents and young adults, who acquire it when they first become sexually active,[24,25] which concurs with the finding in this study that the greatest frequency of infection is in women aged ≤25 years.
HPV infection is a necessary condition for the appearance and development of cervical cancer, but in women aged <25 years, the worldwide incidence of invasive cervical cancer is very low (1.7 per 100,000 women per year). In these women, infection with high-risk HPV and low-grade intraepithelial cervical lesions tend to be transitory phenomena. The mean age of women with high-grade intraepithelial cervical lesions is 30 years, but these may result from infections acquired before that. After this age, it is more difficult for the immune system to clear primary high-risk HPV infection, leaving women at greater risk for developing persistent infections that cause precancerous lesions and invasive cervical cancer.[26]
In some geographic areas, the behavior of cervical cancer has changed due to the increasingly early age of onset of sexual activity and to contagion with high-risk HPV. The consequence of this is appearance of premalignant cervical lesions before the age of 25 years.[27] This change in behavior justifies prevalence or baseline studies for HPV infection in open populations to define the epidemiological situation and the circulation of oncogenic genotypes in different age groups and in different geographical regions of each country.
A meta-analysis that included 1 million women with normal Pap tests from 59 countries and 5 continents reported a prevalence of cervical infection between 1.6% and 25%, with differences among geographic regions, and an overall prevalence of 11.7%. HPV prevalence was 24% in Sub-Saharan Africa, 21.4% in Eastern Europe and 16.1% in Latin America. The lowest prevalence values were found in North America (4.7%) and Western Asia (1.7%).[28] These differences are related to sociodemographic, cultural, epidemiological and clinical aspects.[28] Another meta-analysis, which included 1425 women with normal Pap tests from Australia, Brazil, Canada, Mexico, Saudi Arabia, South Africa, Sweden, Tanzania, Thailand and the USA, found a 12.4% HPV prevalence.[29] We obtained prevalences similar to those in Latin America for women with normal Pap tests, which could be explained by the fact that they are in the same geographical region and share certain sociocultural and epidemiological characteristics.
With regard to genotypes, their distribution may vary among regions within a country, but in general most important is the circulation of high-risk HPV. Depending on which genotypes are circulating, prevention strategies with protective vaccines can be established, taking into account those included in the vaccine formulations available on the market.[30,31] Although genotype distribution was different in the three Cuban provinces, oncogenic genotypes made up the greatest share in all cases, and the most frequent was HPV 16, which is included in commercial vaccines. This result coincides with those for countries in Latin America and throughout the world in women with negative Pap tests, and with cervical lesions of different grades or cervical cancer.[32,33] At least for this genotype, Cuban women would obtain benefits from immunization with imported vaccines until safe and effective vaccine candidates are developed domestically.
Following HPV 16, the next most frequent genotypes differed among the three provinces. Such regional differences are also reported within other countries and geographies. In Brazil;[33] Asia, particularly China;[34,35] and Mexico,[36] the most frequent high-risk HPV genotypes are distinct, which is important both epidemiologically and for vaccine prevention strategies.
Studies in 38 countries (prior to approval and introduction of the Gardasil 9 vaccine) demonstrated that HPV genotypes 16, 18, 31, 33, 45, 52 and 58 contribute considerably to the appearance and development of invasive cervical cancers and other anogenital cancers. Persistent infections with these genotypes are present in approximately 96% of invasive cervical cancer cases and in 70%–90% of all anogenital cancers.[31,37] Concurring with these results, a study of Cuban women in Havana found that HPV genotypes 16, 18, 45 and 58 were associated with presence of high-grade intraepithelial cervical lesions and cervical cancer in women aged >30 years.[9] Another study of 519 women aged 15–59 years in 4 Havana municipalities found that oncogenic genotypes 16 (41.0%), 31 (11.6%) and 18 (10.2%) were the most frequent and were associated with presence of high-grade intraepithelial lesions.[10] In the women in our study with intraepithelial lesions, two high-risk genotypes (31 and 33) were found, which coincide with the ones most frequently identified in the 38 countries, and one (31), with those in the Cuban study of women living in Havana. Although these genotypes are associated with high-grade lesions, they were also present more often in women with negative Pap tests.
 These findings demonstrate that in women with high-grade lesions and cervical cancer, the genotypes of greatest frequency may differ from those identified in the general population and reinforce the positive effect that could result from introduction of commercially-available vaccines in Cuba’s National Immunization Program or introduction of another domestically produced vaccine containing them in its formulation.
These findings demonstrate that in women with high-grade lesions and cervical cancer, the genotypes of greatest frequency may differ from those identified in the general population and reinforce the positive effect that could result from introduction of commercially-available vaccines in Cuba’s National Immunization Program or introduction of another domestically produced vaccine containing them in its formulation.
 High- and low-grade intraepithelial lesions found in the women in this study who had no history of positive Pap tests are associated with HPV infection, which coincides with descriptions in other studies.[29,38,39] Among high-grade intraepithelial lesions, two oncogenic genotypes were detected (66 and 68) that are not contained in the HPV vaccine formulations currently in use.[31,37] Taking into account their association with precancerous cervical lesions in Cuban women, these genotypes could be considered for inclusion in Cuban vaccine candidates.
High- and low-grade intraepithelial lesions found in the women in this study who had no history of positive Pap tests are associated with HPV infection, which coincides with descriptions in other studies.[29,38,39] Among high-grade intraepithelial lesions, two oncogenic genotypes were detected (66 and 68) that are not contained in the HPV vaccine formulations currently in use.[31,37] Taking into account their association with precancerous cervical lesions in Cuban women, these genotypes could be considered for inclusion in Cuban vaccine candidates.
HPV prevalence was similar in the three Cuban provinces, though it was slightly higher in Havana, which could be explained by the sociodemographic profile associated with greater risk of infection. This result differs from those reported by other authors who found greater variations among regions in a single country, as in Brazil and Mexico.[40,41] In-country differences are attributed to social, cultural and economic factors that mediate risk behaviors for acquiring infection.[30,40] Mexico and Brazil, in contrast to Cuba, are countries with diverse ethnic groups and cultural patterns, high rates of poverty, illiteracy and varying levels of health coverage/access, which could influence these quite disparate infection frequencies among states. In comparison, the Cuban population is culturally more homogenous, is covered by a universal health system, and has high literacy and educational levels, facilitating implementation of comparable health promotion and disease prevention actions throughout provinces, although differences between urban and rural areas, for example, cannot be ruled out.
Coinfection with multiple HPV genotypes was more frequent in women living in Havana, probably because this province has the greatest proportion of women aged ≤25 years; HPV infection prevalence studies with broad-spectrum genotyping have demonstrated that multiple infections are more frequent in young women, when they are at the peak of their sexual activity.[33] In coinfection, there is a greater risk of developing precancerous cervical lesions, particularly when viral loads are high (>1000 copies/µL).[42] Coinfection makes it hard to decipher the contribution of individual genotypes to cervical lesion stages, but this is resolved by hierarchically attributing the contribution of each genotype to lesion development, based on the defined prevalence of each genotype in cervical cancer. According to these data, women with cervical cancer who have multiple infections with HPV 16, 18 and 45 may have a poorer disease course and response to treatment.[43,44]
In our study, women aged ≤25 years had greater HPV infection prevalence, which concurs with publications from other countries that report higher infection prevalences in the youngest women, and that infection frequency decreases with increasing age.[45,46] In young women, infection is self-limiting and the histopathological changes are reversible, because at these ages the immune response is efficient and can eliminate the virus from the cells through antiviral effector functions, and tissue repair processes are capable of reversing the cellular changes that produce low-grade lesions.[26]
Although age is important in viral clearing and tissue repair, it is recognized that diverse factors can modify the course of infection, favoring viral persistence and development of cervical cancer. Among these, the most studied are virus-dependent factors, those related to the immune system of infected women and women’s lifestyles. Recognized viral factors include integration of high-risk HPV DNA into the host-cell genome, the expression of viral oncoproteins E6 and E7, and complex interactions between these oncoproteins and proteins p53 and pRb of the infected cell.[47] Mechanisms of immune-system dependent viral persistence are related to defects in cellular immune response, inactivation of interferon synthesis, an increase in expression and liberation of anti-inflammatory cytokines, and deficiencies in the maturation and expression of major histocompatibility complex molecules (that introduce viral antigens into the immune system’s cytotoxic cells) due to action by high-risk HPV oncoproteins.[48] Among lifestyle factors, it has been demonstrated that smoking contributes to viral persistence when the infection is caused by high-risk HPV.[49] Hormonal contraceptive use has also been associated with viral persistence and with HPV-induced carcinogenesis.[50,51]
HPV infection frequency was greater in students, single or reported having had more than three sexual partners. Student status does not seem to be an independent risk factor in itself, because student status probably coincides with young age, singleness and probably higher-risk sexual behavior. Sexual relations with a large number of partners is a risk factor for HPV and other STIs. It has been described that this behavior is frequent in adolescents and young adults, which is why vaccination strategies have focused on immunizing against HPV at early ages, before the onset of sexual activity,[52] but this can be a frequent practice in women of any age, especially single women.
Frequent changes in partners combined with unprotected sex multiplies the risk of contracting HPV. However, this study observed a greater frequency of infection in women who reported condom use. This finding may seem contradictory as it is known that one of the methods for reducing risk of HPV is protected sex. The problem with questions like these, such as condom use and STI history, is that at times patients’ responses do not reflect reality due to social prejudices and perceptions about self-image. For this infection, which is latent and persistent, perhaps the patient uses condoms currently but five or ten years ago did not, or was not using them when she became infected. If condoms had always been used, infection prevalence would most likely be lower.
Although vaccination is the most efficient prophylactic method, because it protects against infection from seven oncogenic HPV genotypes, it is not the only means. Health promotion campaigns in Cuba should more actively promote condom use starting with the onset of sexual relations, because according to the results of this study, oncogenic genotypes that are not included in commercial vaccines are circulating among Cuban women and could infect women exposed to those genotypes, even though it has been reported that cross-protection may exist against some genetically-related genotypes contained in currently available vaccines.[53]
Variables associated with viral infection did not behave equally in the three provinces studied, observing differences primarily in the age of infected women, their occupational status, number of sexual partners in the two years, or smoking and hormonal contraceptive use. The uneven distribution of sociodemographic variables among provinces, particularly those associated with a higher risk of infection, could explain the higher prevalence in Havana as well as the lowest in Villa Clara. For instance, Havana has the highest percentage of women aged 15–25 years, the same range that concentrates higher rates of infection observed in general. It also has the highest percentage of students, of women who reported having three or more sexual partners in the last two years, women with a history of STIs, smokers and oral contraceptive users. It is also true that Havana does not have the greatest percentage of single women, nor those who began sexual relations at age ≤15 years, although it is where the greatest frequencies of infection are seen within these categories by province.
In addition to differences in HPV prevalence among countries and regions and among provinces and cities,[40–54] differences have been reported in sociodemographic and clinical-epidemiological variables associated with HPV infection and cervical cancer between urban and rural areas in a single country.[55,56] These differences also depend on socioeconomic and cultural aspects that modulate the attitudes and behaviors of women living in each region or city.[30,40] Different lifestyles among urban women may influence their susceptibility to HPV infection and persistence. Habits such as smoking, alcohol and drug use, and treatment with steroidal hormones have been related to behaviors associated with infection.[49,50,55] One possible explanation for the differences found among provinces could be that women living in large urban centers such as Havana experience more stress, smoke and drink alcohol more often, are more independent socially, more isolated from their extended families, and have fewer prejudices and religious taboos, and use hormonal contraceptives rather than other methods.[56]
The effects of smoking on HPV infection have been attributed to the benzopyrene in tobacco smoke, which can modulate the lifecycle of HPV, strengthen the expression of viral oncoprotein mRNA and aid in viral persistence, stimulate carcinogenesis and enhance cancer progression.[49] Cigarette smoking has been related to high viral load values for high-risk HPV, and carcinogens in smoke, synergistically with viral infection, may increase risk of progression to cervical cancer.[57] Although at rates lower than men’s, smoking is relatively frequent among Cuban women. In a study of 1959 employees in the education sector, Varona and colleagues found 28.8% of women smoked.[58]
A meta-analysis that studied risk factors for infection and its persistence and for development of cervical cancer reported that long-term oral contraceptive use was an important risk factor.[50] These results are related to the capacity of estrogens to promote virion morphogenesis, viral persistence, tumor growth and positive regulation exercised by estrogens and progesterone on viral oncogenes.[51] In our study, proportions of infection in women who use oral contraceptives were higher than in those who did not, particularly in Havana, but their effect on carcinogenesis was not evaluated because none of the women in the study had cervical cancer lesions.
Chlamydia trachomatis infection was concentrated in women aged ≤25 years, which concurs with worldwide reports of greater prevalence younger women, although figures vary according to region or country: Europe 3.0% to 5.3%, Australia 5.6%, Chile 7.9% and the USA 4.7%.[59–62] In 2012, WHO estimated worldwide chlamydia prevalence in women at 2.4% to 6.9%, highest in the Americas and East Pacific.[63]
Infection prevalence in our study was lower at 1%, which could be due to the fact that mean age of the women was high (38 years) and that the youngest age group was small. Furthermore, this figure does not concur with those from other Cuban studies that assessed chlamydia prevalence in women using molecular techniques, in which values ranged from 6.9% to 8.3%.[15–17] This discrepancy might be attributed to the fact that these studies included women with symptoms of gynecological infections and others who were asymptomatic but who had risk factors associated with HIV infection, while those in our study did not have symptoms, were recruited in primary care when getting a Pap test or other exam, and thus might be considered part of an open population. In most studies globally using these techniques, chlamydia frequencies are significantly greater in symptomatic patients or those who seek care for STIs compared to asymptomatic women and open populations.[64–67]
Chlamydia infection in women is almost always asymptomatic, resulting in late or no diagnosis, which can make transmission easier and also lead to infection chronicity and complications. Thus, we would recommend periodic screening in specific population groups, primarily women aged ≤25 years, those older than 59 years and those with risk factors for infection.[59]
Multiple factors associated with chlamydia infection in women have been described. These include age ≤25 years; being single; and, as with HPV and other STIs, frequent change in sexual partners or having changed partners recently, lack of condom use in recent sexual relations, low socioeconomic level and a history of other STIs. Due to the low frequency of chlamydia infection found, we could not establish associations with the sociodemographic variables in the study design. An important finding was the infrequent circulation of chlamydia in women aged >25 years in the three Cuban provinces, which affirms observations made in daily diagnostic practice of the Pedro Kourí Tropical Medicine Institute’s the STI Laboratory.
Despite the low frequency of chlamydia infection, one woman was found to have coinfection with HPV genotype 61. This result, although isolated, is important since it has been reported that HPV is an essential but not sufficient factor in occurrence of malignant lesions[2] and it has been demonstrated that high-risk HPV genotypes have a central role in cervical cancer pathogeny.[68] Among the endogenous and exogenous cofactors explaining the occurrence of malignant lesions in high-risk HPV infections, chlamydia coinfection stands out, since it is the most frequent and most widely distributed bacterial STI, and has been associated with occurrence of malignant or precancerous cervical lesions.[69–72] One biological explanation for the role of coinfection is that chlamydia causes loss of epithelial integrity thus facilitating HPV access to basal epithelial layers, increasing viral persistence and progression of chlamydia infection. It is also postulated that chlamydia infection can induce chronic inflammation, interfere with immune response by decreasing the number of antigen-presenting cells, and reduce cellular immunity to foster HPV persistence.[72]
It is important to underscore that this study is limited to findings related to infection frequency in three regional scenarios, and to describing and evaluating their association with sociodemographic, clinical and epidemiological variables, and that the sampling design used, based on women seen in primary care services, does not allow for inferences to be made at a population scale.
CONCLUSIONS
Women living in three Cuban provinces have a high frequency of HPV infection with proportions higher for high-risk genotypes. HPV 16 is the most frequent genotype. Other circulating genotypes vary by geographic region. Younger women have a greater risk of HPV infection; they have more frequent coinfection with several HPV genotypes, at least one of which is of high oncogenic risk. Although frequency of high-grade cervical lesions is low, these are associated with a high percentage of infection by oncogenic HPV genotypes, but none of the women infected with high-risk HPV had chlamydia.
The prevalence of chlamydia infection is low; only the youngest women were infected and, within this study, coinfection with HPV was not associated with presence of cervical lesions.
ACKNOWLEDGMENTS
The authors thank the health authorities and the Provincial Hygiene, Epidemiology and Microbiology Centers (CPHEM) in Havana, Villa Clara and Holguín Provinces, for their collaboration in clinical sampling and data gathering, as well as the women who participated in this study.
References

- World Health Organization [Internet]. Geneva: World Health Organization; c2019. Centro de Prensa. Infecciones de transmisión sexual. Datos y cifras; 2019 Jun 14 [cited 2019 Sep 3]. Available from: http://www.who.int/mediacentre/factsheets/fs110/es/. Spanish.
- zur Hausen H. Papillomaviruses in the causation of human cancers – a brief historical account. Virology Feb 20 [Internet]. 2009 [cited 2019 Sep 5];384(2):260–5. Available from: https://www.sciencedirect.com/science/article/pii/S0042682208007721?via%3Dihub
- Centre on HPV and Cancer. Human Papillomavirus and Related Diseases Report. Cuba. ICO Information [Internet]. Washington, D.C.: Centre on HPV and Cancer; 2018 [cited 2019 Sep 9]. Available from: https://hpvcentre.net/statistics/reports/CUB_FS.pdf
- de Villiers EM. Cross-roads in the classification of papillomaviruses. Virology [Internet]. 2013 Oct [cited 2019 Sep 9];445(1–2):2–10. Available from: https://www.clinicalkey.es/service/content/pdf/watermarked/1-s2.0-S0042682213002456.pdf?locale=es_ES&searchIndex=
- Rader JS, Tsaih SW, Fullin D, Murray MW, Iden M, Zimmermann MT, et al. Genetic variations in human papillomavirus and cervical cancer outcomes. Int J Cancer [Internet]. 2019 May 1 [cited 2019 Sep 9];144(9):2206–14. Available from: https://onlinelibrary.wiley.com/resolve/doi?DOI=10.1002/ijc.32038
- Santana Serrano C, Chávez Roque M, Viñas Sifontes LN, Hernández López E, Cruz Pérez J. Diagnóstico precoz del cáncer cérvicouterino. Ginecol Salud Reprod [Internet]. 1999 [cited 2019 Sep 9]; [about 4 screens]. Available from: http://www.bvs.sld.cu/revistas/gin/vol37_2_11/gin11211.htm
- National Health Statistics and Medical Records Division (CU). Anuario Estadístico de Salud 2018 [Internet]. Havana: Ministry of Public Health (CU); 2019 [cited 2019 Sep 9]. 197 p. Available from: http://files.sld.cu/bvscuba/files/2019/04/Anuario-Electr%C3%B3nico-Espa%C3%B1ol-2018-ed-2019-compressed.pdf Spanish
- Franceschi S, Herrero R, Clifford GM, Snijders PJF, Arslan A, Anh PTH, et al. Variations in the age-specific curves of human papillomavirus prevalence in women worldwide. Int J Cancer [Internet]. 2006 Dec 1 [cited 2019 Sep 10];119(11):2677–84. Available from: https://onlinelibrary.wiley.com/doi/pdf/10.1002/ijc.22241
- Soto Y, Torres G, Kourí V, Limia CM, Goicolea A, Capo V, et al. Molecular epidemiology of human papillomavirus infections in cervical samples from Cuban women older than 30 years. J Low Genit Tract Dis [Internet]. 2014 Jul [cited 2019 Sep 10];18(3):210–7. Available from: http://insights.ovid.com/pubmed?pmid=24270200
- Soto Brito Y, Limia León CM, Kourí Cardellá V, Goicolea Maiza A, Capó de Paz V, Muné Jiménez M. Papilomavirus humanos y otros factores asociados al desarrollo de lesiones cervicouterinas en mujeres cubanas. Panorama Cuba y Salud [Internet]. 2016 Jan–Apr [cited 2019 Sep 10];11(1):24–33. Available from: https://www.redalyc.org/pdf/4773/477355397005.pdf. Spanish.
- Unemo M, Bradshaw CS, Hocking JS, de Vries HJC, Francis SC, Mabey D, et al. Sexually transmitted infections: challenges ahead. Lancet Infect Dis [Internet]. 2017 Aug [cited 2019 Sep 10];17(8):e235–79. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1473-3099(17)30310-9
- Llaguno Concha AA. Factores socio epidemiológicos y clínicos presentes en mujeres atendidas en consulta de infertilidad. Rev Cubana Ginecol Obstet [Internet]. 2015 [cited 2019 Sep 10];41(4):365–75. Available from: http://scielo.sld.cu/pdf/gin/v41n4/gin06415.pdf. Spanish.
- Granado-Martínez O, Figueroa-Mendoza M, Almaguer-Almaguer JA, López-Artze O, Arroyo-Díaz Y, Gutiérrez-Díaz M, et al. Cirugía de mínimo acceso en la infertilidad femenina. Rev Cubana Ginecol Obstet [Internet]. 2010 [cited 2019 Sep 10];36(3):368–81. Available from: http://scielo.sld.cu/pdf/gin/v36n3/gin08310.pdf. Spanish.
- Álvarez Miranda MC, Martín Raimundo D, Jiménez Puñales S, Pentón Cortes RJ, Cairo González VM. Cirugía tubárica endoscópica: una alternativa para el manejo de la mujer infértil en Villa Clara. Acta Médica Centro [Internet]. 2013 [cited 2019 Sep 10];7(4). Available from: http://www.revactamedicacentro.sld.cu/index.php/amc/article/view/21/165. Spanish.
- Rivero-Figueroa D, Kourí-Cardellá V, Correa-Sierra C, Martínez-Mota I, Llanes Caballero R. Barreal González RT. Detección de Chlamydia trachomatis en muestras de exudado endocervical mediante una prueba de diagnóstico rápido y dos técnicas de reacción en cadena de la polimerasa. Rev Cubana Ginecol Obstet [Internet]. 2014 [cited 2019 Sep 10];40(1):48–57. Available from: http://scielo.sld.cu/pdf/gin/v40n1/gin06114.pdf. Spanish.
- Frontela Noda M, Rodríguez Marín Y, Verdejas Varela OL, Valdés Martínez FJ. Infección por Chlamydia trachomatis en mujeres cubanas en edad reproductiva. Rev Cubana Endocrinol [Internet]. 2006 [cited 2019 Sep 10];17(2). Available from: http://scielo.sld.cu/pdf/end/v17n2/end01206.pdf. Spanish.
- Kourí V, Cartaya J, Rodríguez ME, Muné M, Soto Y, Resik S, et al. Prevalence of Chlamydia trachomatis in human immunodeficiency virus-infected women in Cuba. Mem Inst Oswaldo Cruz [Internet]. 2002 Dec [cited 2019 Sep 10];97(8):1073–7. Available from: http://www.scielo.br/pdf/mioc/v97n8/4511.pdf
- Papanicolaou GN. A new procedure for staining vaginal smears. Science [Internet]. 1942 Apr 24 [cited 2019 Sep 11];95(2469):438–9. Available from: http://www.sciencemag.org/cgi/pmidlookup?view=long&pmid=17842594
- Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, et al. A review of human carcinogens–Part B: biological agents. Lancet Oncol [Internet]. 2009 Apr [cited 2019 Sep11];10(4):321–2. Available from: https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(09)70096-8/fulltext
- Wei HB, Zou SX, Yang XL, Yang DQ, Chen XD. Development of multiplex real-time quantitative PCR for simultaneous detection of Chlamydia trachomatis and Ureaplasma parvum. Clin Biochem [Internet]. 2012 Jun [cited 2019 Sep 11];45(9):663–7. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0009-9120(12)00138-5
- World Medical Association. World Medical Association. Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA [Internet]. 2013 Nov 27 [cited 2019 Sep 11];310(20):2191–4. Available from: https://jamanetwork.com/journals/jama/fullarticle/1760318?appId=scweb
- Van Delden JJM, Van der Graaf R. Revised CIOMS International Ethical Guidelines for health-related research involving humans. JAMA [Internet]. 2017 Jan 10 [cited Sep 11]; 317(2):135–6. Available from: https://jamanetwork.com/journals/jama/article-abstract/2592245
- Nyitray AG, Iannacone MR. The epidemiology of human papillomaviruses. Curr Probl Dermatol [Internet]. 2014 [cited Sep 12];45:75–91. Available from: https://www.karger.com/Article/PDF/358370
- Dunne EF, Unger ER, Sternberg M, McQuillan G, Swan DC, Patel SS, et al. Prevalence of HPV infection among females in the United States. JAMA [Internet]. 2007 Feb 28 [cited 2019 Sep 12];297(8):813–9. Available from: https://jamanetwork.com/journals/jama/fullarticle/10.1001/jama.297.8.813
- Weinstock H, Berman S, Cates W Jr. Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health [Internet]. 2004 Jan–Feb [cited 2019 Sep 12];36(1):6–10. Available from: https://www.guttmacher.org/pubs/journals/3600604.html
- Howell-Jones R, de Silva N, Akpan M, Oakeshott P, Carder C, Coupland L, et al. Prevalence of human papillomavirus (HPV) infections in sexually active adolescents and young women in England, prior to widespread HPV immunisation. Vaccine [Internet]. 2012 Jun 6 [cited 2019 Sep 12];30(26):3867–75. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0264-410X(12)00525-7
- Baldauf JJ, Fender M, Akladios CY, Velten M. [Is early cervical cancer screening justified?]. Gynecol Obstet Fertil [Internet]. 2011 [cited 2019 Sep 12];39(6):358–63. Available from: https://www.ncbi.nlm.nih.gov/pubmed/21600827. French.
- Bruni L, Díaz M, Castellsagué X, Ferrer E, Bosch FX, de Sanjosé S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis [Internet]. 2010 Dec 15 [cited 2019 Sep 12];202(12):1789–99. Available from: https://academic.oup.com/jid/article-lookup/doi/10.1086/657321
- Aguilar-Lemarroy A, Vallejo-Ruiz V, Cortés-Gutiérrez EI, Salgado-Bernabé ME, Ramos-González NP, Ortega-Cervantes L, et al. Human papillomavirus infections in Mexican women with normal cytology, precancerous lesions, and cervical cancer: type-specific prevalence and HPV coinfections. J Med Virol [Internet]. 2015 May [cited 2019 Sep 12];87(5):871–84. Available from: https://doi.org/10.1002/jmv.24099
- Villa LL. Overview of the clinical development and results of a quadrivalent HPV (types 6, 11, 16, 18) vaccine. Int J Infect Dis [Internet]. 2007 Nov [cited 2019 Sep 13];11 Suppl 2:S17–25. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1201-9712(07)60017-4
- Joura EA, Giuliano AR, Iversen OE, Bouchard C, Mao C, Mehlsen J, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med [Internet]. 2015 Feb 19 [cited 2019 Sep 13];372(8):711–23. Available from: https://www.nejm.org/doi/10.1056/NEJMoa1405044?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dwww.ncbi.nlm.nih.gov
- Carozzi F, De Marco L, Gillio-Tos A, Del Mistro A, Girlando S, Baboci L, et al. Age and geographic variability of human papillomavirus high-risk genotype distribution in a large unvaccinated population and of vaccination impact on HPV prevalence. J Clin Virol [Internet]. 2014 Jul [cited 2019 Sep 15];60(3):257–63. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1386-6532(14)00142-5
- Bruno A, Serravalle K, Travassos AG, Lima BG. [Genotype distribution of human papillomavirus in women from the state of Bahia, Brazil]. Rev Bras Ginecol Obstet [Internet]. 2014 Sep [cited 2019 Sep 15];36(9):416–22. Available from: http://www.scielo.br/pdf/rbgo/v36n9/0100-7203-rbgo-36-09-00416.pdf. Portuguese.
- Wang XC, Sun LQ, Ma L, Li HX, Wang XL, Wang X, et al. Prevalence and genotype distribution of human papillomavirus among women from Henan, China. Asian Pac J Cancer Prev [Internet]. 2014 [cited 2019 Sep 15];15(17):7333–6. Available from: http://journal.waocp.org/article_29769_cf3b31b6ca1f24bf246ee346c8c698ca.pdf
- Sun B, He J, Chen X, He M, He Z, Wang Y, et al. Prevalence and genotype distribution of human papillomavirus infection in Harbin, Northeast China. Arch Virol [Internet]. 2014 May [cited 2019 Sep 15];159(5):1027–32. Available from: https://link.springer.com/content/pdf/10.1007%2Fs00705-013-1886-1.pdf
- Ortega-Cervantes L, Aguilar-Lemarroy A, Rojas-García AE, Barrón-Vivanco BS, Vallejo-Ruiz V, Cantú de León D, et al. Human papilloma virus genotypes in women from Nayarit, Mexico, with squamous intraepithelial lesions and cervical cancer. Int J Health Sci (Qassim) [Internet]. 2016 Jul–Sep [cited 2019 Sep 15];10(3):327–38. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5003576/pdf/ijhs-10-3-327.pdf
- Serrano B, de Sanjosé S, Tous S, Quiros B, Muñoz N, Bosch X, et al. Human papillomavirus genotype attribution for HPVs 6, 11, 16, 18, 31, 33, 45, 52 and 58 in female anogenital lesions. Eur J Cancer [Internet]. 2015 Sep [cited 2019 Sep 15];51(13):1732–41. Available from: http://incan-mexico.org/wp_ginecologia/wp-content/uploads/Human-papillomavirus-genotype-attribution-in-female-anogenital-lesions.pdf
- Tabrizi SN, Brotherton JM, Stevens MP, Condon JR, McIntyre P, Smith D, et al. HPV genotype prevalence in Australian women undergoing routine cervical screening by cytology status prior to implementation of an HPV vaccination program. J Clin Virol [Internet]. 2014 Jul [cited 2019 Sep 15];60(3):250–6. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1386-6532(14)00146-2
- Mejía L, Muñoz D, Trueba G, Tinoco L, Zapata S. Prevalence of human papillomavirus types in cervical cancerous and precancerous lesions of Ecuadorian women. J Med Virol [Internet]. 2016 Jan [cited 2019 Sep 15];88(1):144–52. Available from: https://onlinelibrary.wiley.com/doi/pdf/10.1002/jmv.24310
- Coser J, da Rocha Boeira T, Simon D, Kazantzi Fonseca AS, Ikuta N, Lunge VR. Prevalence and genotypic diversity of cervical human papillomavirus infection among women from an urban center in Brazil. Genet Mol Res [Internet]. 2013 Feb 19 [cited 2019 Sep 12];12(4):4276–85. Available from: http://www.geneticsmr.com/articles/2101
- Flores-Miramontes MG, Torres-Reyes LA, Aguilar-Lemarroy A, Vallejo-Ruíz V, Piña-Sánchez P, Cortés-Gutiérrez E, et al. [HPV genotypes prevalence in Mexico and worldwide detected by Linear Array]. Rev Med Inst Mex Seguro Soc [Internet]. 2015 [cited 2019 Sep 12];53 Suppl 2:S122–30. Available from: https://www.medigraphic.com/pdfs/imss/im-2015/ims152c.pdf. Spanish.
- Wang SM, Colombara D, Shi JF, Zhao FH, Li J, Chen F, et al. Six-year regression and progression of cervical lesions of different human papillomavirus viral loads in varied histological diagnoses. Int J Gynecol Cancer [Internet]. 2013 May [cited 2019 Sep 15];23(4):716–23. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3636161/pdf/nihms444305.pdf
- Wentzensen N, Wilson LE, Wheeler CM, Carreon JD, Gravitt PE, Schiffman M, et al. Hierarchical clustering of human papilloma virus genotype patterns in the ASCUS-LSIL triage study. Cancer Res [Internet]. 2010 Nov 1 [cited 2019 Sep 15];70(21):8578–86. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/20959485/
- Schmitt M, Depuydt C, Benoy I, Bogers J, Antoine J, Arbyn M, et al. Multiple human papillomavirus infections with high viral loads are associated with cervical lesions but do not differentiate grades of cervical abnormalities. J Clin Microbiol [Internet]. 2013 May [cited 2019 Sep 15];51(5):1458–64. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3647930/pdf/zjm1458.pdf
- de Sanjosé S, Wheeler CM, Quint WGV, Hunt WC, Joste NE, Alemany L, et al. Age-specific occurrence of HPV16- and HPV18-related cervical cancer. Cancer Epidemiol Biomarkers Prev [Internet]. 2013 Jul [cited 2019 Sep 15];22(7):1313–8. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4306595/pdf/nihms-648924.pdf
- Monsonego J, Zerat L, Syrjanen K, Zerat JC, Smith JS, Halfon P. [Prevalence of genotype-specific HPV infection among women in France: implications for screening and vaccination]. Gynecol Obstet Fertil [Internet]. 2013 May [cited 2019 Sep 15];41(5):305–13. Available from: https://www.sciencedirect.com/science/article/pii/S1297958913000878?via%3Dihub. French.
- Díaz D, Santander MA, Chavez JA. HPV-16 E6 and E7 oncogene expression is downregulated as a result of Mdm2 knockdown. Int J Oncol [Internet]. 2012 Jul [cited 2019 Sep 15];41(1):141–6. Available from: https://www.spandidos-publications.com/ijo/41/1/141
- Bodily J, Laimins LA. Persistence of human papillomavirus infection: keys to malignant progression. Trends Microbiol [Internet]. 2011 Jan [cited 2019 Sep 15];19(1):33–9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3059725/pdf/nihms245474.pdf
- Alam S, Conway MJ, Chen HS, Meyers C. The cigarette smoke carcinogen benzo[a]pyrene enhances human papillomavirus synthesis. J Virol [Internet]. 2008 Jan [cited 2019 Sep 16];82(2):1053–8. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/17989183/
- Vinodhini K, Shanmughapriya S, Das BC, Natarajaseenivasan K. Prevalence and risk factors of HPV infection among women from various provinces of the world. Arch Gynecol Obstet [Internet]. 2012 Mar [cited 2019 Sep 16];285(3):771–7. Available from: https://dx.doi.org/10.1007/s00404-011-2155-8
- Marks M, Gravitt PE, Gupta SB, Liaw KL, Kim E, Tadesse A, et al. The association of hormonal contraceptive use and HPV prevalence. Int J Cancer [Internet]. 2011 Jun 15[cited 2019 Sep 16];128(12):2962–70. Available from: https://doi.org/10.1002/ijc.25628
- Rysavy MB, Kresowik JD, Liu D, Mains L, Lessard M, Ryan GL. Human papillomavirus vaccination and sexual behavior in young women. J Pediatr Adolesc Gynecol [Internet]. 2014 Apr [cited 2019 Sep 16];27(2):67–71. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1083-3188(13)00280-5
- Malagon T, Drolet M, Boily MC, Franco EL, Jit M, Brisson J, et al. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis [Internet]. 2012 Oct [cited 2019 Sep 16];12(10):781–9. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1473-3099(12)70187-1
- Girianelli VR, Thuler LCS, e Silva GA. [Prevalence of HPV infection among women covered by the family health program in the Baixada Fluminense, Rio de Janeiro, Brazil]. Rev Bras Ginecol Obstet [Internet]. 2010 Jan [cited 2019 Sep 12];32(1):39–46. Available from: https://www.semanticscholar.org/paper/%5BPrevalence-of-HPV-infection-among-women-covered-by-Girianelli-Thuler/95ed4458cd963a7498d55eec6d4a567eebb9bec8
- Zahnd WE, Rodríguez C, Jenkins WD. Rural-urban differences in human papillomavirus-associated cancer trends and rates. J Rural Health [Internet]. 2019 Mar [cited 2019 Sep 16];35(2):208–15. Available from: https://onlinelibrary.wiley.com/doi/pdf/10.1111/jrh.12305
- Raychaudhuri S, Mandal S. Socio-demographic and behavioural risk factors for cervical cancer and knowledge, attitude and practice in rural and urban areas of North Bengal, India. Asian Pac J Cancer Prev [Internet]. 2012 [cited 2019 Sep 16];13(4):1093–6. Available from: http://journal.waocp.org/article_26298_e73d70143926839642144a3724161d93.pdf
- Wei L, Griego AM, Chu M, Ozbun MA. Tobacco exposure results in increased E6 and E7 oncogene expression, DNA damage and mutation rates in cells maintaining episomal human papillomavirus 16 genomes. Carcinogenesis [Internet]. 2014 Oct [cited 2019 Sep 16];35(10):2373–81. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4178472/pdf/bgu156.pdf
- Varona P, García RG, Garcia RM, Lorenzo E. Tabaquismo y percepción del riesgo de fumar en trabajadores de la educación. Rev Cub Salud Pública 2016;42(1):45–60. Available from: https://www.scielosp.org/article/rcsp/2016.v42n1/o6/
- Wiesenfeld HC. Screening for Chlamydia trachomatis infections in women. N Engl J Med [Internet]. 2017 Feb 23 [cited 2019 Sep 16];376(8):765–73. Available from: http://www.nejm.org/doi/full/10.1056/NEJMcp1412935?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed
- Redmond SM, Alexander-Kisslig K, Woodhall SC, van den Broek IV, van Bergen J, Ward H, et al. Genital chlamydia prevalence in Europe and non-European high income countries: systematic review and meta-analysis. PLoS One [Internet]. 2015 Jan 23 [cited 2019 Sep 16];10(1):e0115753. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4304822/pdf/pone.0115753.pdf
- Martínez MA, Reid SI, Arias C, Napolitano RC, Sandoval ZJ, Molina CR. Prevalence of cervical infection by Chlamydia trachomatis among Chilean women living in the Metropolitan Region. Rev Med Chil [Internet]. 2008 [cited 2019 Sep 16];136(10):1294–300. Available from: https://scielo.conicyt.cl/pdf/rmc/v136n10/art09.pdf. Spanish.
- Torrone E, Papp J, Weinstock H; Center for Disease Control and Prevention (CDC). Prevalence of Chlamydia trachomatis genital infection among persons aged 14-39 years–United States, 2007-2012. MMWR Morb Mortal Wkly Rep [Internet]. 2014 Sep 26 [cited 2019 Sep 16];63(38):834–8. Available from: https://www.cdc.gov/mmwr/pdf/wk/mm6338.pdf
- Newman L, Rowley J, Vander Hoorn S, Wijesooriya NS, Unemo M, Low N, et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One [Internet]. 2015 Dec 8 [cited 2019 Sep 16];10(12):e0143304. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4672879/pdf/pone.0143304.pdf
- Adams EJ, Charlett A, Edmunds WJ, Hughes G. Chlamydia trachomatis in the United Kingdom: a systematic review and analysis of prevalence studies. Sex Transm Infect [Internet]. 2004 Oct [cited 2019 Sep 17];80(5):354–62. Available from: https://sti.bmj.com/content/sextrans/80/5/354.full.pdf
- Underhill G, Hewitt G, McLean L, Randall S, Tobin J, Harindra V. Who has chlamydia? The prevalence of genital tract Chlamydia trachomatis within Portsmouth and South East Hampshire, UK. J Fam Plann Reprod Health Care [Internet]. 2003 Jan [cited 2019 Sep 17];29(1):17–20. Available from: https://srh.bmj.com/content/familyplanning/29/1/17.full.pdf
- Mascellino MT, Ciardi MR, Oliva A, Cecinato F, Hassemer MP, Borgese L. Chlamydia trachomatis detection in a population of asymptomatic and symptomatic women: correlation with the presence of serological markers for this infection. New Microbiol [Internet]. 2008 Apr [cited 2019 Sep 16];31(2):249–56. Available from: http://www.newmicrobiologica.org/PUB/allegati_pdf/2008/2/249.pdf
- Thomas P, Spaargaren J, Kant R, Lawrence R, Dayal A, Lal JA, et al. Burden of Chlamydia trachomatis in India: a systematic literature review. Pathog Dis [Internet]. 2017 Jul 31[cited 2019 Sep 17];75(5). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5808648/pdf/ftx055.pdf
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer [Internet]. 2015 Mar 1[cited 2019 Sep 17];136(5):359–86. Available from: https://onlinelibrary.wiley.com/doi/pdf/10.1002/ijc.29210
- Zhu H, Shen Z, Luo H, Zhang W, Zhu X. Chlamydia trachomatis infection-associated risk of cervical cancer: a meta-analysis. Medicine (Baltimore) [Internet]. 2016 Mar [cited 2019 Sep 17];95(13):e3077. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4998531/pdf/medi-95-e3077.pdf
- Jensen KE, Thomsen LT, Schmiedel S, Frederiksen K, Norrild B, van den Brule A, et al. Chlamydia trachomatis and risk of cervical intraepithelial neoplasia grade 3 or worse in women with persistent human papillomavirus infection: a cohort study. Sex Transm Infect [Internet]. 2014 Nov [cited 2019 Sep 17];90(7):550–5. Available from: https://sti.bmj.com/content/90/7/550.long
- Luostarinen T, Namujju PB, Merikukka M, Dillner J, Hakulinen T, Koskela P, et al. Order of HPV/Chlamydia infections and cervical high-grade precancer risk: a case-cohort study. Int J Cancer [Internet]. 2013 Oct 1 [cited 2019 Sep 17];133(7):1756–9. Available from: https://onlinelibrary.wiley.com/doi/pdf/10.1002/ijc.28173
- Karim S, Souho T, Benlemlih M, Bennani B. Cervical cancer induction enhancement potential of Chlamydia trachomatis: a systematic review. Curr Microbiol [Internet]. 2018 Dec [cited 2019 Sep 17];75(12):1667–74. Available from: https://link.springer.com/article/10.1007%2Fs00284-018-1439-7
THE AUTHORS
Elías Guilarte-García, physician with dual specialities in family medicine and microbology with a master’s degree in virology. Sexually-Transmitted Infections Laboratory, Pedro Kourí Tropical Medicine Institute (IPK), Havana, Cuba.
Yudira Soto-Brito (Corresponding author: yudira@ipk.sld.cu), microbiologist with a doctorate in health sciences and a master’s degree in virology. Senior researcher, Sexually-Transmitted Infections Laboratory, IPK, Havana, Cuba.
Vivian Kouri-Cardellá, physician specializing in microbiology with a master’s degree in virology and infectious diseases, and doctorats in medical sciences and sciences. Senior researcher, Sexually-Transmitted Infections Laboratory, IPK, Havana, Cuba.
Celia Maria Limia-León, biochemist with a master’s degree in virology. Adjunct researcher, Sexually-Transmitted Infections Laboratory, IPK, Havana, Cuba.
Maria de Lourdes Sánchez-Alvarez, physician specializing in microbiology with a master’s degree in infectious diseases. Adjunct researcher, Villa Clara Provincial Hygiene, Epidemiology and Microbiology Center, Santa Clara, Cuba.
Ana Elisa Rodríguez-Díaz, clinical bioanalyst with a master’s degree in parasitology. Pathological Anatomy Department, IPK, Havana, Cuba.
Ledy X. López-Fuentes, histopathologist with a master’s degree in bacteriology/mycology, Pathological Anatomy Department, IPK, Havana, Cuba.
Melisa Méndez-González, microbiologist, Sexually-Transmitted Infections Laboratory, IPK, Havana, Cuba.
Nancy Aróstica-Valdés, physician specializing in obstetrics and gynecology, Marta Abreu Polyclinic, Santa Clara, Cuba.
Marlevis Bello-Pérez, physician with dual specialties in family medicine and epidemiology. Villa Clara Provincial Hygiene, Epidemiology and Microbiology Center, Santa Clara, Cuba.
Lissette Pérez-Santos, microbiologist with a doctorate in health sciences and a master’s degree in virology. Senior researcher, Sexually-Transmitted Infections Laboratory, IPK, Havana, Cuba.
Yanet Pintos-Saavedra, microbiologist. Sexually-Transmitted Infections Laboratory, IPK, Havana, Cuba.
Yoanna Baños-Morales, senior technician for science, health, and the environment, Sexually-Transmitted Infections Laboratory, IPK, Havana, Cuba.
Submitted: March 11, 2020 Approved: January 20, 2020 Disclosures: None








