INTRODUCTION
Of the 130 million children born annually worldwide, an estimated 0.78 million to 3.9 million are preterm neonates weighing < 1500 g and are thus considered very low birth weight (VLBW) infants.[1] Incidence of VLBW infants in developed countries is 0.6%–3%.[1–3]
Unprecedented international advances in technology and pharmacology have transformed neonatal intensive care, particularly in recent decades, leading to increases in VLBW infants survival at levels that seem harder and harder to exceed. Survival rate for very low birth weight infants in developed countries ranges between 80% and 85%,[4] which coincides with Cuba’s VLBW neonatal survival rate in 1989–2004.[5] Morbidity and sequelae over time, however, mainly related to neurodevelopment, remain a challenge for clinical neonatology.[6,7] It is increasingly important to study VLBW infants and provide longitudinal followup after hospital discharge, to ensure early diagnosis of neurodevelopment abnormalities, enabling timely intervention for better quality of life.[8,9]
Since all surviving VLBW infants will grow up in their social environment, these children’s performance should be examined in their family and social settings. Various factors (such as prematurity, very low birth weight, maternal morbidity, and neonatal morbidity with associated intensive care) can adversely influence their neurodevelopment; hence the importance of studying longterm neurologic prognosis.[5] In addition to IQ and severe disorders (cerebral palsy, epilepsy, severe neurosensory impairments), incidence of more minor abnormalities, such as attention deficit and mild behavioral disorders, should also be assessed.[9]
Havana’s Dr Ramón González Coro University Maternity Hospital (HMURGC) is a reference center for care of VLBW infants in Cuba’s capital. Traditionally, pregnant women at high risk for giving birth to infants weighing < 1500 g have sought treatment there because, in addition to personnel specialized in perinatology, HMURGC also has the necessary resources to provide comprehensive care. These factors explain why, in the five-year period 2006–2010, proportion of VLBW births at HMURGC (1%)[10] was higher than in the rest of the country (0.6%).[11]
During recent decades we have focused on providing care for these children and meeting the challenge of their survival and quality of life. This study was undertaken to review neurodevelopment outcomes over the first two years of life of a group of VLBW infants born at HMURGC in 2006–2010, and to correlate abnormalities detected with the children’s weight, gestational age, and Apgar scores (both 1-minute and 5-minute) at birth.
METHODS
Type of study and patients A case-series study was conducted to assess neurodevelopment of VLBW infants in Havana’s HMURGC during 2006–2010. Over the study period, 132 VLBW infants were born, of whom 87.9% (116) survived to age two years, conforming the study population. The surviving infants’ neurodevelopment was monitored, concluding with a final examination at corrected age of 2 years, defined as the age the child would be if he/she had been born full term (40 weeks of gestational age). This is the method commonly used to monitor this type of patient up to 24 months.[8]
Variables
Birth weight Each infant’s birth weight (in the first hour of life) was recorded using a scale (Atom, Japan) with weighing error of 5 g; absolute values were used and the subjects were grouped in three categories: < 1000 g, 1000–1249 g, and 1250–1499 g.
Gestational age at birth Gestational age at birth (in weeks) was recorded, starting from the first day of the mother’s last menstrual cycle. Two groups were formed: < 30 weeks and ≥30 weeks.
Apgar score at one minute The Apgar score at one minute after birth was recorded and data separated into two groups: < 7 points and ≥7 points.
Apgar score at five minutes The Apgar score at five minutes after birth was recorded and data separated into two groups: <7 points and ≥7 points.
Independent variable: neurodevelopment At age two years corrected, patients were classified (neurologic examination described below) according to HMURGC neurodevelopment unit followup protocol.
Normal. No neurodevelopmental abnormality.
Mild abnormalities. Mild or transient muscular hypotonia, mild motor impairment with hypertonia, mild or transient reflex abnormalities, transient or mild psychomotor delay, discrete or transient hypertonia, mild mental delay, hyperactivity, mild language delay.
Moderate-to-severe abnormalities. Moderate-to-severe hypo-tonia without personal or social adjustment problems, moderate-to-severe psychomotor delay, moderate-to-severe mental delay, moderate-to-severe language delay, hyperkinetic syndrome, spastic cerebral palsy or chronic cerebral motor or sensory impairment, hydrocephaly, microcephaly, epilepsy, severe retinopathy of prematurity. A diagnosis of cerebral palsy was considered confirmed if characteristic clinical signs (such as motor impairment of cerebral origin) were present at the end of one year corrected age.
Neurologic examination Each patient underwent a traditional neurologic assessment at 40±2 weeks of corrected age, as well as a polysomnography with an electroencephalograph (Medicid 5, Neuronic SA, Cuba) with electrodes placed according to the international system for a standard bipolar montage.[12] Multidisciplinary neurodevelopmental monitoring of all subjects was conducted from hospital discharge to two years corrected age with at least six checkups. The HMURGC neurodevelopment unit protocol was applied, which includes clinical signs (the Amiel-Tison neurologic assessment[13] in the first year and classic neurologic examination in the second year), psychological tests (Bayley scales of mental and motor development),[14] morphological tests (imaging studies such as transfontanellar ultrasound series, ALOKA equipment, Japan), and cranial computed axial tomography (Phillips tomography, Netherlands) as needed, as well as various neurophysiological tests (brainstem auditory evoked potentials and visual evoked potentials) to examine electrocerebral activity and neurosensory functioning.
Data collection and analysis A general data collection form for each subject was designed and completed with updated data on the variables studied. This information was then entered into a Microsoft Excel 2010 database. Descriptive measures such as absolute values, percentages and arithmetic means were used.
To identify possible relationships between perinatal variables and neurodevelopment, Pearson’s chi-square test of independence was applied, using the exact sampling distribution and a significance threshold of p < 0.05. Variables were grouped by categories; that is, patients with normal neurodevelopment and patients with abnormalities (which included mild and moderate-to-severe). All data were processed using Microsoft Excel 2010 and Windows SPSS 11.5. The database used a Windows 7 platform.
Ethices Written informed consent was obtained from each patient’s parents. Patient anonymity was maintained in data analysis. The HMURGC ethics committee approved the study.
RESULTS
One hundred sixteen (116) surviving patients were assessed, with zero attrition. Average gestational age at birth was 31.5 weeks, and average birth weight was 1295.1 g.
Of the 116 VLBW infants, 69% developed normally, 25.9% showed mild neurodevelopmental abnormalities, and 5.2% displayed moderate-to-severe neurodevelopmental abnormalities (Table 1). Of the 36 neonates with neurodevelopmental abnormalities (31% of total), 6 had moderate or severe abnormalities and 30 mild abnormalities; 18 children (15.5% of total) had mild or transient abnormalities of muscle tone and 16 (13.8%) showed mild delays in language or psychomotor development (Table 1).
Table 1: Neurodevelopment and abnormalities detected in the first two years of life of VLBW infants (n = 116)

*A patient could have >1 abnormality / VLBW: very low birth weight
There was a statistically significant relationship between gestational age at birth and neurodevelopment in the first two years of corrected age (p = 0.006). Of children born at < 30 weeks’ gestation, 22.2% presented moderate-to-severe abnormalities vs. 2% of children born at ≥30 weeks (Table 2).
No statistically significant relationship was observed between any of the variables of birth weight (p = 0.448), 1-minute Apgar score (p = 0.42) and 5-minute Apgar score (p = 0.999) and neurodevelopment (Table 2).
DISCUSSION
The survival rate of VLBW infants (87.9%) was higher than that observed by Tsou in similar patients in Taiwan (76.2%)[15] or Fernández-Carrocera in Mexico City (50%).[16] In contrast, Kono reported an even higher rate (almost 92%) in Japan, a highly developed country.[17]
In her 15-year comprehensive followup study (1989–2004) of 200 surviving neonates discharged from HMURGC, Gessesse found only 47.5% of VLBW children had completely normal neurodevelopment,[5] compared with 69% in our study. A combination of factors may have contributed to this improvement: the maternal/fetal medical service set up in our center for providing enhanced perinatal care for at-risk pregnancies; the purchase of modern neonatal respiratory equipment for neonatal care, and ongoing professional development of the staff in charge of VLBW neonatal care. We found several studies with similar results in the international literature reviewed. In Germany, Moll reported 75% of VLBW neonates had normal neurological development[18] and, in Italy, Orcesi reported that 83.4% of his patients developed normally during the first 24 months of corrected age, a higher figure than that found in our study.[19]
Table 2: Neurodevelopment in the first two years of life of VLBW infants related to perinatal variables
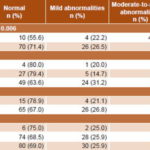
VLBW: very low birth weight
The level of moderate-to-severe abnormalities reported by Gessesse was twice that of our study (11% vs. 5.2%), and incidence of mild abnormalities was substantially higher (41.5% vs. 25.9%).[5] These differences may be explained by the institutional improvements already mentioned. On the other hand, Orcesi reported lower incidence of mild alterations (10.9%) compared with our study, but similar incidence (5.1%) of moderate-to-severe abnormalities.[19]
Our results differ from other studies with respect to the most common type of mild abnormalities found (mild or transient muscle tone abnormalities and slight delays in language and psychomotor development). For example, Gessesse reported hyperactivity to be the most common,[5] and Sangtawesin (Thailand) reported psychomotor development disorders as the predominant mild abnormality.[20]
Comparing incidence of some moderate-to-severe abnormalities (cerebral palsy, retinopathy of prematurity, and deafness) with results reported by Gessesse,[5] Moll,[18] Mukhopadhyay[21] and Ballot,[22] we found very similar rates for infantile cerebral palsy, varying from 1% to 4% (2.6% in our study). Concerning neurosensory auditory impairment, Moll reports an incidence of < 1%,[18] compared with 0.9% in our study. In contrast, Sangtawesin[20] reported 3.3% severe auditory impairment. With regard to retinopathy of prematurity, the 1.7% rate we observed was well below the 25.4% reported by Ali in Brunei[23] but similar to the 1.1% reported by Reyes in Mexico.[24]
The statistically significant relationship that we found between gestational age and neurodevelopment (the younger
the gestational age at birth, the greater the probability of neurodevelopmental abnormalities) coincides with Serenius’s findings in Sweden;[25] he reported that patients born at ≥30 weeks’ gestational age had higher rates of normal neurodevelopment than those born at < 30 weeks. Filipouski in Brazil and Zoban in the Czech Republic show similar results.[26,27]
Our study did not find direct relationship between birth weight and normal neurodevelopment expected according to the international literature, including research by Stoinska, which found lower birth weight associated with higher incidence of abnormalities,[28] and Kwinta’s study, which associated birth weight of < 1000 g with more severe neurodevelopmental disorders.[29] It is worth noting that the latter was a followup study up to age seven years, while ours was up to age two years.
This short time limited our research since patients must be followed over a longer period to confirm or rule out longterm neurodevelopmental abnormalities—for example, those noticeable only after children start school.
Our results did not support the generally held view that sustained lower Apgar scores are associated with increased risk of neurodevelopmental abnormalities.[30,31] None of our patients with low Apgar scores at either one or five minutes showed more than mild neurodevelopmental abnormalities. In his study, Ehrenstein proposed that absolute risk of neurologic abnormalities in low-Apgar-score survivors is low and that low Apgar score alone is a poor clinical predictor of longterm neurodevelopment.[32]
Although other Cuban institutions have made isolated efforts to examine the magnitude of neurodevelopmental sequelae in VLBW infants, national statistics are still unavailable. Multicenter studies are urgently needed, given the rising incidence of VLBW (0.6%–1%) in national reference hospitals caring for such neonates,[10,33,34] and in recent international publications, which report incidence rates up to 1.26%.[35,36]
A further limitation of our study was the small number of patients, which could have influenced results. However, although the population was not very large, the strength of our study is that 100% of survivors underwent a thorough assessment, which is rare in the published literature. These results could provide a useful baseline for a similar but larger-scale longer study continuing through school age. It is critically important to understand VLBW infants’ neurodevelopment and to conduct early interventions to ensure better quality of life in this steadily-growing patient group.
CONCLUSION
Very low birth weight infants born at < 30 weeks gestational age who survive face a higher risk for developing neurodevelopmental abnormalities.





