INTRODUCTION
In recent decades, chronic kidney disease (CKD) has become a serious global health problem, its epidemiological picture revealing substantial and even alarming increases in both incidence and prevalence. The number of patients with end-stage renal disease (ESRD) continues to grow rapidly,[1] requiring major investment of resources in medical care. Despite better management of dialysis patients, morbidity and mortality continue to rise. Thus, identification, prevention and control of CKD risk factors before, at initiation of, and during dialysis are important for patient outcomes, population health and health systems.[2–4]
The increasing prevalence of CKD reflects the increasing prevalence of its leading causes, such as diabetes and hypertension, as well as with worldwide trends in aging. Thus, early in CKD progression, control of underlying causes is key, but in later stages, renal replacement therapies become necessary.
Cuba has a CKD prevention program that covers the whole population through family physicians and community nephrologists (integrated with secondary and tertiary care for dialysis and kidney transplant patients), which contributes to decreasing late diagnosis of this disease, a problem everywhere. Early diagnosis aids comprehensive patient care prior to dialysis and kidney transplantation and improves patient survival.[2,5,6]
Although specifics are not completely known for all factors predictive of hemodialysis patient survival, the influence of many of them on patient progress is known. Some, such as diabetes, are causes of ESRD; others include advanced age, late diagnosis and referral to nephrologist, malnutrition, and vascular access problems. It is important to identify these factors in order to develop comprehensive risk reduction strategies to facilitate channeling health resources and activities to their prevention and treatment.[7]
The objective of this study was to identify prognostic factors in ESRD patients on hemodialysis.
METHODS
A descriptive, longitudinal, study was done of 81 ESRD patients receiving periodic hemodialysis at the Medical-Surgical Research Center (CIMEQ, the Spanish acronym) in Havana from January 1995 through December 2004. Each admission to hemodialysis was considered one case, for a replacement therapy population of 96, since 15 patients returned to hemodialysis after transplant failure and were considered new cases for purposes of this analysis.
Included were end stage, or stage 5, CKD patients (dialysis-dependent ESRD patients) aged ≥15 years who had been in a hemodialysis program for at least three months. Excluded were patients on temporary hemodialysis or who had been receiving treatment for less than three months, those for whom hemodialysis was permanently discontinued, and patients whose treatment began with peritoneal dialysis or who were transferred to peritoneal dialysis. Patient data were recorded from initiation of hemodialysis until the end of the study, transfer to renal transplant therapy or death.
The following variables were analyzed:
- Age
- Sex
- Causes of ESRD (hypertension; diabetes; chronic glomerular diseases; polycystic kidneys; urological disorders, such as vesicoureteral reflux, obstructive uropathy and kidney stones; vasculitis; idiopathic causes)
- Late referral (first consult with nephrologist less than nine months before initiation of replacement therapy)[6]
- Malnutrition (body mass index, BMI <18 kg/m2 at beginning of hemodialysis and/or during last six months before end of treatment or end of study)[8]
- Hypoalbuminemia (serum albumin <3.5 g/dL at beginning of hemodialysis and/or during six months before end of treatment or end of study)[9]
- Uncontrolled hypertension (blood pressure ≥140/90 at beginning of hemodialysis and/or at over 50% of monitoring checkups during hemodialysis followup)[10]
- Anemia (hemoglobin <11 g/dL at beginning of hemodialysis and/or at over 50% of monitoring checkups during hemodialysis followup)[11]
- Inadequate vascular access (absence of arteriovenous fistula or vascular graft, or ones that did not permit sufficient flow for hemodialysis)
- Cardiovascular disease (including left ventricular hypertrophy, angina, myocardial infarction and heart failure)
- Diabetes (according to clinical diagnosis based on fasting hyperglycemia recorded in patient histories)[12]
- Chronic liver disease (generally caused by hepatitis B or C virus)
- Inadequate diet and/or fluid intake (intake of more food and liquids than appropriate causing hydrosaline overload and electrolyte disorders requiring modification of dialysis strategy; weight gain >6%)
- Survival
- Underlying causes of death of patients who died during study period
Analysis Information was processed using SPSS version 13.0 statistical software. Summary descriptive measures were used: mean and standard deviation for quantitative variables, and percentage for qualitative variables. The chi-square test was used for comparison of qualitative variables and t test for quantitative variable means. The McNemar test was used to compare presence of prognostic factors at initiation of and during dialysis treatment, significance level set at p < 0.05. The Kaplan Meier method was used for survival analysis. A log-rank test was used to compare different levels of a single variable. A Cox regression model was fitted to assess risk of death for a given prognostic factor (presence or worsening during the study).
Ethics This was a noninterventional descriptive study based on analysis of hemodialysis patient records, without treatment modification. Patient identification information was kept confidential. The project was approved by CIMEQ’s ethics committee and scientific advisory council.
RESULTS
Mean patient age was 40.9 ± 12.9 years; there were no age differences by sex. Most subjects were men (60/81, 74.1%).
Only 6.2% of patients had late referral to a nephrologist. The leading causes of ESRD were hypertension and diabetes, in 25% (24/96) and 21.9% (21/96) of cases, respectively; followed by glomerular disease (20.8%, 20/96), polycystic kidney (18.8%,18/96), urological conditions (9.4%, 9/96), and vasculitis (1%, 1/96). Some (3.1%, 3/96) of ESRD cases were of unknown etiology.
Factors present in patients at initiation of and during dialysis treatment are listed in Table 1. Considerable uncontrolled hypertension and anemia were observed at treatment initiation. There were substantial increases in chronic liver disease, malnutrition, hypoalbuminemia and cardiovascular diseases over the course of treatment, while there was a significant decrease in uncontrolled hypertension.
Table 1: Prognostic factors in ESRD patients at initiation and duringa hemodialysis, 1995–2004 (n = 96)
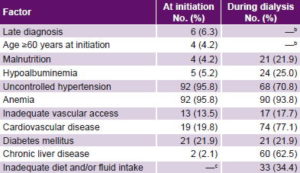
a At any point during survival on dialysis b Not susceptible to change during dialysis c Not applicable prior to dialysis ESRD: end-stage renal disease
Mean hemodialysis patient survival at the end of the study period was 4.4 years, (95% CI: 2.2–5.3 years). At one year, survival was 88.6%, falling to 54.7% at three years and 26.6% at five years (Figure 1).
Figure 1: Cumulative survival of ESRD patients on hemodialysis, 1995–2004

ESRD: end-stage renal disease
Prognostic factors In univariate analysis using Kaplan Meier survival curves, factors significantly increasing risk of death were hypertension (p = 0.001), inadequate vascular access (p < 0.001), diabetes (p = 0.014) and inadequate diet and fluid intake (p = 0.029). Factors predictive of death according to Cox regression analysis were uncontrolled hypertension, RR 6.57 (CI 1.09–39.28, p = 0.039), inadequate vascular access, RR 4.66 (CI 1.63–13.37, p = 0.004) and diabetes, RR 2.84 (CI 1.15–7.02, p = 0.023) (Table 2).
Table 2: Hemodialysis patient survival by prognostic factor, 1995–2004

a by five years, all surviving patients had developed cardiovascular disease
Table 3: Underlying cause of death, associated vascular disease in ESRD patients on hemodialysis, 1995–2004 (n = 31)
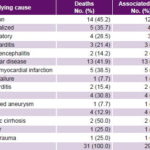
CVD: cardiovascular disease ESRD: end-stage renal disease
Underlying causes of death in hemodialysis patients were infections in 45.2% (14/31) and cardiovascular diseases in 41.9% (13/31). In the latter group, acute myocardial infarction predominated (38.5%, 5/13). Other causes accounted for 12.9% of deaths (cirrhosis of the liver, cancer and head trauma). Regardless of underlying cause of death, 93.5% of those who died had cardiovascular disease (Table 3).
DISCUSSION
The majority of hemodialysis patients in this study were men, congruent with studies in Europe.[13] It has been suggested that men with glomerular disease may have worse prognosis, but there is no conclusive evidence that sex is a determining factor in CKD progression.[13,14]
Hypertension, diabetes and glomerular diseases were the leading causes of CKD in these hemodialysis patients, similar to reports from other studies.[14,15] Hypertension is a cause, consequence and aggravating factor of CKD, and is highly prevalent in hemodialysis patients. Hypertension is often determined to be the cause of CKD by exclusion of other causes; defining hypertension as causal without histological confirmation of nephroangiosclerosis may lead to overestimation. This might constitute bias and be a study limitation, resulting from decisions common in medical practice to dispense with renal biopsy in these patients, since biopsy benefits may not outweigh risks.[16] Diabetic nephropathy is, along with hypertension, a major cause of CKD, consistent with our findings. An alarming global increase has been reported in incidence of diabetic nephropathy in type 2 diabetes, progressively displacing glomerular diseases among the main CKD etiologies.[16]
The late nephrology referral rate seen in our patients is a satisfactory finding when compared with reports ranging from 25% to 50%.[17] One of the advantages of early diagnosis is to ensure adequate vascular access, indispensable for successful dialysis. This is consistent with our finding that the majority in this series had adequate vascular access.
In this series, 4.2% of cases were malnourished at dialysis initiation, a percentage that increased perceptibly during hemodialysis. Malnutrition can coincide with development of CKD due to the influence of underlying causes, and has been reported in 40% to 50% of patients at dialysis initiation,[18] proportions much higher than in our sample, for reasons that deserve further investigation. The causes of malnutrition are many: among them, appetite suppressants, certain socioeconomic and cultural conditions, comorbidity and depression. Hormonal imbalances associated with CKD—peripheral insulin resistance, secondary hyperparathyroidism and low levels of growth hormone—are conducive to malnutrition.[19] It is important to clarify that BMI was used to evaluate nutritional status, as has been done by others,[20] without analyzing other aspects, such as anthropomorphic variables, biochemical imbalances, body composition and diet. Thus, this could result in underreporting of malnutrition in patients before and during dialysis, a study limitation to bear in mind for future research.
In addition to the abovementioned factors, others can lead to impaired nutrition in dialysis patients, including dialysis technique, nutrient loss during dialysis (water-soluble vitamins, amino acids and peptides) and the nausea and vomiting that frequently accompany hemodialysis. Furthermore, blood contact with the dialysis membrane triggers an inflammatory response that stimulates protein catabolism; response intensity depends on membrane composition, and is more pronounced with cellulose membranes.[21]
We found no statistically significant effect of serum albumin values on survival, possibly a result of small sample size. Serum albumin is a biochemical marker that can decrease modestly with the reduced protein and calorie intake associated with uremic syndrome, without significant comorbidity or elevated proinflammatory cytokines; this imbalance is known as type 1 malnutrition. In other cases, hypoalbuminemia can be substantial and be associated with elevated resting energy expenditure, increased oxidative stress and protein metabolism, and there can also be considerable comorbidity and elevated concentrations of proinflammatory cytokines; this state is known as type 2 malnutrition.[22]
Uncontrolled hypertension decreased dramatically over the course of dialysis treatment, although it continued to be a problem, as reported for other series.[22] This implies that in addition to volume overload, other mechanisms must influence pathogenesis of hypertension. The possible mechanisms implicated are related to increase in peripheral vascular resistance. It is known that worldwide some 80% to 90% of patients enter dialysis with hypertension, which does not seem to be controlled with the dialysis treatment they normally receive. In some patients, blood pressure can rise during a hemodialysis session as a paradoxical side effect of ultrafiltration, due to the renin-angiotensin-aldosterone system reaction to excessive volume loss.[23]
Anemia showed few changes over the course of treatment, maintaining a prevalence of 93.8%. This may be because our patients did not receive erythropoietin systematically until the end of the study; anemia is common in ESRD patients who are not receiving erythropoietin, and our levels may have remained high because the drug was not available until the end of the study period. In dialysis patients, anemia can considerably affect various organs and systems, primarily the cardiovascular and endocrine systems and cognitive function.[24] It has been shown that for each g/dL increase in hemoglobin, relative risk of death decreases by 5% and of hospitalization by 4%.[25,26] Despite this, we found no significant effect of anemia, and the proportion of anemic patients did not change appreciably over the study.
Inadequate vascular access for hemodialysis in this series was a prognostic factor that influenced survival. These results concur with reports from other authors, in which the greatest risk of mortality is related to more frequent infections in patients with suboptimal vascular access.[27]
The high prevalence of CVD in ESRD patients is well established, and it is the leading cause of morbidity and mortality in hemodialysis. It is reported that only 16% of patients enter hemodialysis with a normal echocardiogram, 65% have left ventricular hypertrophy, 41% have ischemic heart disease and 40% have heart failure.[28] Our study found that there was a substantial increase in CVD during dialysis therapy.
Besides the well-known traditional contributors to CVD pathogenesis, dialysis patients have additional ones, including anemia, a hyperdynamic state induced by the arteriovenous fistula, secondary hyperparathyroidism, hyperphosphatemia and malnutrition. These factors do not entirely explain increased CVD in this population; and in recent years there has been reference to new, nontraditional factors that must be involved, such as oxidative stress and systemic inflammatory response.[29]
The considerable CVD increase we observed during dialysis may be related to underreporting before dialysis initiation. Additionally, absence of symptoms revealing this disease may have impeded heart disease diagnosis in the predialysis stage. Early diagnosis of CKD could enable earlier detection of CVD.
The observed negative effect of CVD on survival grew more marked over time and was most dramatic at five years, when there were no longer any survivors without CVD. Death from a cardiovascular cause is more frequent among dialysis patients and even more so in those who are diabetic and elderly.[30]
Analysis of patient survival with diabetes as the prognostic factor showed significant differences. When diabetics begin renal replacement therapy, they present greater comorbidity, greater frequency of heart failure, peripheral vascular disease and chronic liver disease. Vascular causes of death, primarily cardiovascular causes, are also the most frequent among diabetic patients on maintenance dialysis.[31–33]
A high prevalence liver disease was observed among patients in our study, even in the context of the high rates in the dialysis population globally.[16] Our results may reflect greater use of blood transfusions prior to the time when erythropoietin became available. Notably, only 25% of our patients received erythropoietin in 2000, increasing progressively to 100% coverage reached by the study’s end. Measures to prevent transmission of hepatitis C virus continue to be vitally important.
In renal replacement therapy, patient survival is undoubtedly the most important question. Dialysis technique has been shown to influence survival;[34] also important is the correct prescription and adjustment of dialysis, ensuring sessions are long enough to meet patient needs.[23] Other influential factors associated with the need for or conditions of dialysis include dialysis center experience, adherence to therapy, and comorbidities such as CVD and diabetes.[34] It has been noted that the most common causes of death are, in general, more closely related to these associated factors than to the technique itself. In fact, comorbidities—principally CVD and diabetes—prior to hemodialysis initiation are a key determinant of prognosis and patient survival.[34,35] These aspects were not addressed in the present study and should be explored in future research.
Our findings that infections and CVD are the primary underlying causes of death are similar to those of other reports, in which causes of vascular origin were found in up to 50% of cases, with infections in second place (the latter the most frequent cause in patients aged 20–44 years).[36,37] Potentially fatal infections are 50 times more frequent in these patients than in the general population.[38]
CONCLUSIONS
The probable negative effect of prognostic factors, particularly CVD and infections, on morbidity and survival of ESRD patients in dialysis makes prevention, identification and control of these conditions critically important for optimal patient survival.







