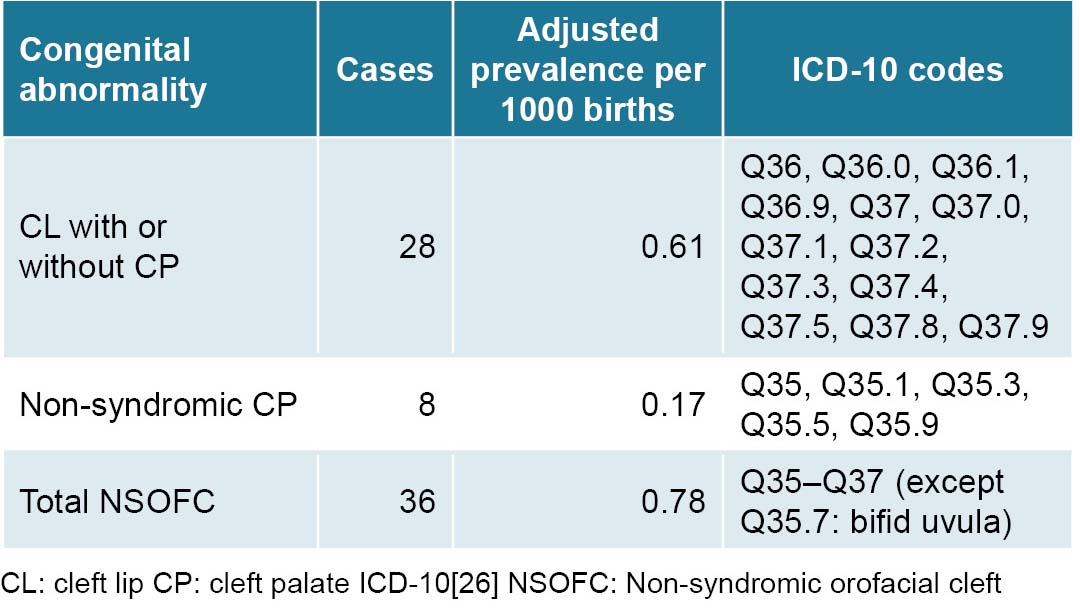
In several national and international studies, isolated CP represents only a third to a half of all observed NSOFC cases.[8,24,34,36] Other studies show even lower frequencies, including one carried out in Villa Clara Province in the 1990s at the José L. Miranda Pediatric University Hospital’s orthodontic service, where isolated CP represented only 11.4% of all NSOFC cases.[37]
One possible explanation for this difference is that hard-palate fissures are frequently accompanied by fissures in the lips.[8] Additionally, the lip and palate have different embryological origins: the palate is derived from the endodermal lamina, while the lips are derived from the ectodermal lamina.[2]
Adjusted NSOFC prevalence at birth in Villa Clara Province (0.78 per 1000 births) is within the range described both in Cuba and worldwide (0.7–1.5 per 1000 newborns).[2,22,29] This frequency is slightly higher than that found in research on congenital disorders in newborns carried out in Havana from 2000 to 2003, in which CL with or without CP NSOFC represented 5.4% of all isolated congenital abnormalities, at a prevalence of 0.71 per 1000 births.[38] This coincides with that observed by Cisneros in Santiago de Cuba, who diagnosed 98 NSOFC cases in 138,381 births over a period of 10 years.[34]
However, over a five-year period (2007–2011), researchers in Havana found an adjusted CL (with or without CP) prevalence rate of 1.1 per 1000 births in Arroyo Naranjo municipality,[39] lower than observed frequencies in the years 1998 and 2000 in Octavio de la Concepción de la Pedraja Pediatric University Hospital in Holguín, a province in eastern Cuba, at 1.22 and 1.26 per 1000 live births, respectively.[40]
Frequency of congenital malformations varies greatly by ethnicity: populations of African ancestry have the lowest prevalence, followed by those of European descent, and then populations of Native American and Asian ancestry, who have the highest prevalence.[6–8,10]
A descriptive analysis using ECLAMC clinical-epidemiological data from 9 South American countries for 1995–2012, identified 2773 newborns with NSOFC (2293 with CL with or without CP, and 480 with isolated cleft palate), for an overall prevalence of 1.08 per 1000 newborns.[20]
In Guadalajara, Mexico, which has a large indigenous population, a 2009–2016 study found a high prevalence of NSOFCs (2.79 per 1000).[41] Other authors studying NSOFC rates in Mexico have estimated overall frequencies between 0.6–0.9 per 1000 live births.[28] In the USA, NSOFC rates vary considerably among different ethnic groups, with higher frequencies among Asian Americans and Native Americans (2 per 1000), followed by non-Hispanic whites and Hispanics (1–1.43 per 1000), and with lowest frequencies among African Americans (0.4 per 1000).[7]
In addition to genetic factors that influence NSOFC-frequency differences among ethnic groups, economic and sociocultural factors play a role in NSOFC spatial variation. For example, NSOFC rates vary within ethnic groups and nationalities in Africa, from 0.5 per 1000 in Ghana and Nigeria (the latter the most populous African country and the nation with the largest Black population worldwide, at >160 million people); 0.7 per 1000 in Malawi; 1.4 per 1000 in Ethiopia; and up to 1.65 per 1000 in Kenya.[21,42]
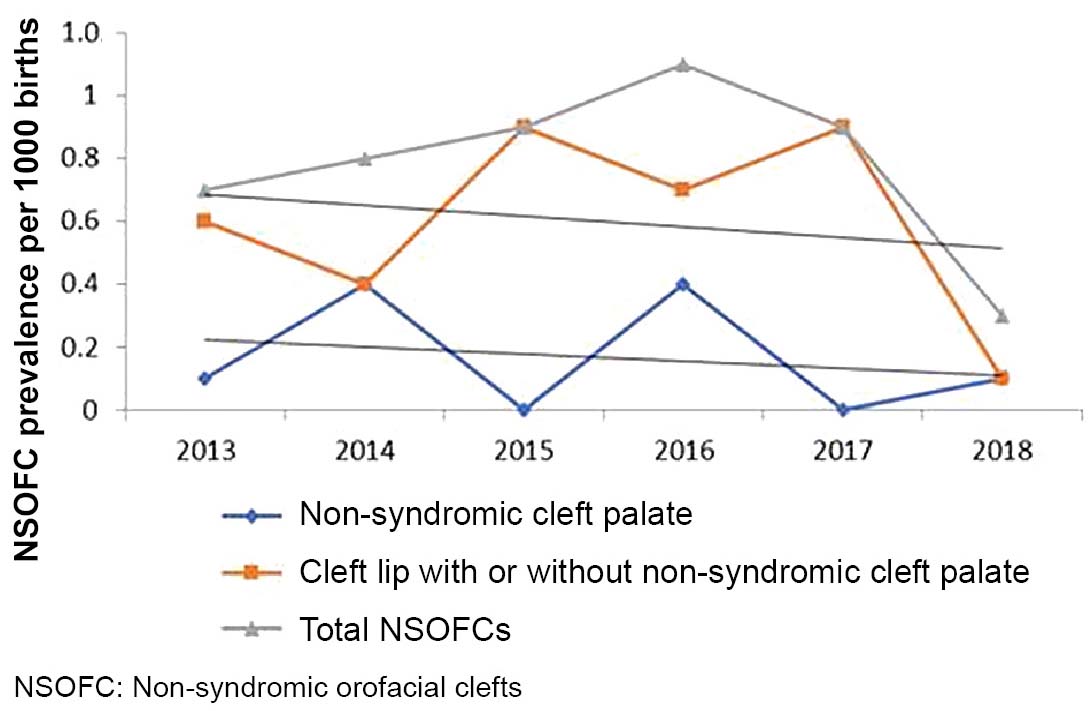
A recent meta-analysis estimated NSOFC prevalence at 1.38 per 1000 births in low- and middle-income countries.[35] Risk factors are often interrelated; for example, Chileans with higher NSOFC rates are in the lowest socioeconomic strata and are also more likely to be of indigenous ancestry.[5] Possible explanations for differences in prevalence among geographic regions and socioeconomic statuses include environmental factors like vitamin intake, nutrition, access to health care and education and lifestyle-related factors, like smoking and alcohol abuse.[36,43] The decrease in cases after 2016 in our study could be attributed, in part, to increasing consumption of folic acid. In mid-2015, Villa Clara province implemented a program promoting preconceptive use of folic acid supplements in all women of childbearing age.
Spatiotemporal variability analysis using spatial epidemiology techniques can offer important clues as to environmental disease etiologies. Numerous geospatial cluster studies have been conducted examining the effect of environmental factors on different congenital abnormalities.[16,20,28]
A primary spatiotemporal NSOFC cluster was identified in two municipalities in the extreme northwest corner of Villa Clara Province in 2017. Secondary spatiotemporal clusters were observed in the northern municipalities of Cifuentes and Encrucijada between 2013 and 2016, which merits further study.
These findings are similar to others that have identified geospatial variations for this type of congenital abnormality. For example, there are significant differences in NSOFC prevalence in different regions of China, with higher prevalence in the country’s interior (frequencies of 3.0–4.7 per 1000) compared to coastal areas (at frequencies of 0.98–1.29 per 1000).
Researchers believe that environmental pollution, economic conditions, health service coverage and diagnostic capacity could contribute to geographic variation.[8] Even within the same Chinese province, great spatial variability has been reported; as an example, NSOFC prevalence in Jiayuguan (3.9 per 1000) and Dingxi (2.71 per 1000) were higher than other cities in Gansu Province. In that case, researchers did not observe significant correlations between economic conditions and NSOFC prevalence.[44]
Descriptive analysis of high NSOFC prevalence in South America found a spatial NSOFC cluster encompassing two cities in southern Ecuador (Cañar and Azogues). Different genetic and environmental risk factors were associated with NSOFCs in this area, including advanced maternal and paternal age, parental consanguinity, low maternal education level and use of drugs such as antimicrobials in the first trimester of pregnancy.[20]
Other NSOFC risk factors are low serum zinc concentrations and inadequate maternal levels of folic acid and other vitamins. In fact, according to WHO, 94% of congenital abnormalities occur in developing countries, where mothers are more vulnerable to malnutrition. In particular, micronutrients have a decisive influence on the health of pregnant women and normal fetal and placental development.[15,45,46] Zinc is an important micronutrient in embryo-fetal development and zinc deficiency is linked to nonsyndromic CP and other congenital abnormalities in animal studies.[21]
An exploratory ecological study of NSOFC spatial variability in the metropolitan area of Monterrey, Mexico, showed a marked concentration of cases in the urban periphery that resulted in several spatial clusters in the northeast territory showing a strong spatiotemporal association with environmental pollution.[28]
Although NSOFC spatiotemporal analyses do not offer causal explanations for identified clusters, they often generate working hypotheses that can be tested in future research. More targeted research designs, such as population-based case-control studies, should be used to explore potential causes of NSOFC clusters in Villa Clara Province. This study lays the foundation for future studies of specific NSOFC risk factors, including alcoholic beverage consumption; smoking; nutritional disorders, including deficiencies in zinc, folic acid, or other micronutrients; and heavy metal and nitrate levels in the soil and in drinking water in the territories under study.
Identifying NSOFC causal factors is the first step to NSOFC primary prevention. This study’s strength is the high reliability and coverage level of the Cuban prenatal and postnatal records of congenital abnormalities, as well as absence of unregistered NSOFCs due to the network of community genetics services located in every municipality, whose counselors evaluate all newborns at one and three months of age. This study’s limitations are similar to those of all spatial analysis research, including biases related to extrapolating aggregate-level associations to the individual scale, as well as expected limitations of spatiotemporal analyses, namely the inability to provide causal explanations for the study’s results.
CONCLUSIONS
NSOFC frequency in Villa Clara Province, Cuba from 2013–2018 fell within expected Cuban and worldwide prevalence ranges. NSOFCs increased during the first four years of the study and began decreasing in 2016. Our research identified primary and secondary spatiotemporal NSOFC clusters in several geographically close municipalities. Although the underlying cause of this clustering remains unknown, identification of these clusters allows for more targeted investigations aimed at pinpointing factors related to NSOFC etiology.
ACKNOWLEDGMENTS
The authors thank Dr Gisela Noche and Dr Ana E. Algora from the Villa Clara Provincial Medical Genetics Center for their assistance in obtaining RECUMAC and RECUPREMAC data, respectively. We also thank the genetics counselors of Villa Clara Province’s 13 municipalities, who assess all newborns at one month and three months of age in primary healthcare centers and contribute to the countrywide congenital abnormality registry.