ABSTRACT
INTRODUCTION Disease and deaths from HIV/AIDS have decreased since antiretroviral treatment was introduced in 1996. Since 2005, as treatment availability has increased worldwide, deaths from HIV/AIDS have declined 48%. As of November 2019, 26,952 cases have been reported in Cuba, of which 5159 (19.1%) are deceased. The country has experienced a reduction in mortality rates since 2002, when antiretroviral treatment became available. Although there are clearly benefits to treatment, it is important to understand antiretroviral safety profiles as their toxicity may lower treatment adherence.
OBJECTIVE Describe adverse reactions attributable to antiretrovirals used in Cuban patients living with HIV/AIDS.
METHODS I studied notifications of adverse reactions to antiretrovirals used in Cuban patients with HIV/AIDS from January 2003 to December 2017. The sample consisted of 352 notifications in the National Pharmacovigilance Database regarding adverse reactions attributed to antiretrovirals. The variables considered were sex, notification year, antiretroviral drug, and number, type, frequency and severity of adverse reactions, whether or not they were preventable, and the reasons for categorizing them as they were.
RESULTS Antiretrovirals reported an average adverse reaction rate of 2.1 per million population per year, representing 24.2% of adverse reactions produced by the antiviral drug group in that period. Adult males represented 75% (264/352) of patients who had adverse reactions to antiretrovirals. Most adverse reactions were in response to nevirapine (29.0%; 102/352) and zidovudine (26.7%; 94/352). The most frequent reactions were hypersensitivity (24.4%; 86/352), digestive disorders (15.9%; 56/352) and anemia (15.6%; 55/352). Reactions were common (62.5%; 220/352) and moderate in severity (70.4%; 248/352). Preventable reactions made up 52.6% (185/352) of adverse reactions. Of preventable reactions, 68.1% (126/185) were associated with drug interactions and 16.2% (30/185) with improper dosage or prescription errors.
CONCLUSIONS Adverse reactions to antiretrovirals in Cuban patients are common and moderate in severity. The drug with the most notifications was nevirapine, and the most common adverse reaction was hypersensitivity. More than half of adverse reactions are considered preventable, and their main causes are prescription errors.
KEYWORDS Drug-related side effects and adverse reactions, antiretroviral agents, secondary prevention, tertiary prevention, Cuba
INTRODUCTION
HIV is a major global health problem. By the end of 2019, 75.7 million people had contracted HIV since the beginning of the epidemic, and 32.7 million died of AIDS-related causes. In 2019, 1.7 million persons were infected with the virus, despite global campaigns to reduce transmission. The introduction of antiretroviral therapy has reduced deaths and contributed to the perception of HIV/AIDS as a chronic disease.[1]
Effective treatment to achieve maximum, prolonged plasma viral load suppression is based on early individualized use of a combination of antiretroviral drugs administered sequentially.[1] Therapy should include at least three antiretrovirals with at least two different mechanisms of action. Introduction of this treatment regime led to radical changes in the infection’s natural history, although over time it became clear that the main limitation of antiretrovirals was their toxicity.[2] Because they are taken indefinitely, long-term toxicity increases, adherence decreases, and patients leave treatment, which fosters both the appearance and transmission of resistant strains.[3]
IMPORTANCE
This study updates the safety profile of antiretrovirals used in Cuban patients living with HIV/AIDS, children of seropositive mothers, and those accidentally exposed to the virus. Profiles are important for improving treatment regimens by reconfiguring the therapeutic combinations recommended in international standards for specific patients. Safety profiles also provide information for personalized treatment.
Timely detection of HIV infection allows early treatment, which reduces illness and death from AIDS. Starting antiretroviral treatment upon diagnosis reduces deaths,[4] gives infected persons a life expectancy similar to that of uninfected persons, and lowers transmission rates in couples with one uninfected person. One of the main obstacles to effective response to the HIV/AIDS epidemic is late diagnosis.[4,5]
From 2010 to 2019, new HIV infections worldwide decreased by 23% in adults and 52% in children. Antiretrovirals capable of reducing viral loads to zero played an essential role in this reduction, as did increased protective measures. Until June 2020, only 26 million infected people had access to antiretroviral therapy, or about 68% of HIV-positive individuals worldwide. Since their 2004 peak, deaths from the virus have dropped more than 60%. However, many people in low- and middle-income nations cannot access antiretrovirals, although WHO and the UN have proposed 2030 as the year of zero AIDS-related deaths, zero new infections and zero discrimination against people living with HIV/AIDS.[5–8]
As of November 2019, 26,952 cases of HIV had been reported in Cuba. By the end of that year, prevalence was 0.4% in people aged 15–49 years, the lowest in the region, and one of the lowest in the world. AIDS was responsible for 89.9% of the 5159 deaths from HIV.[9]
Treatment with domestically-produced generic antiretrovirals began in mid-2001 as part of a national strategy to ensure full treatment access for HIV/AIDS patients. Cuban Therapeutic Guidelines were updated in 2002.[9] These advised that treatment should begin as soon as a person is diagnosed as seropositive, based on clinical, immunologic and virologic criteria established by the scientific community.[9] When Cuban generics were introduced, infection-related health indicators changed because of the clinical efficacy of antiretrovirals.[10] The number of deaths and opportunistic infections greatly decreased, survival increased, treatment became more accessible, and patient quality of life improved.[11] By 2003, the Cuban health system guaranteed access and free treatment to 100% of HIV-positive patients. Manufacturing generic drugs in-country that replaced imports increased therapeutic benefits and allowed potential savings of US$46 million in the first decade that they were produced.[12,13]
The antiretrovirals currently used are divided into several subgroups: nucleoside reverse transcriptase inhibitors (NRTIs: stavudine, lamivudine, zidovudine, abacavir), non-nucleoside reverse transcriptase inhibitors (NNRTIs: nevirapine, efavirenz), protease inhibitors (indinavir, ritonavir, saquinavir), entry inhibitors (also called fusion inhibitors: enfuvirtide), integrase inhibitors (raltegravir, elvitegravir, dolutegravir), and coreceptor antagonists (maraviroc).
Most adverse reactions to these drugs are tolerable, although some may be serious and show drug group effects common to antiretrovirals with the same mechanism of action. Nucleoside reverse transcriptase inhibitors produce mitochondrial damage and protease inhibitors cause metabolic changes, while non-nucleoside reverse transcriptase inhibitors do not have these effects. Adverse reactions in nonnucleoside reverse transcriptase inhibitors are drug-specific and can range from variable-intensity hypersensitivity reactions to neurological disorders. Hypersensitivity is caused by an immune response to some element of the drug and can provoke reactions as varied as skin lesions, to Stevens–Johnson syndrome, epidermal necrolysis, eosinophilia and other systemic reactions.[14,15] In general, treatment benefits outweigh the risk of adverse reactions. As understanding of drug safety profiles increases, treatment protocols improve, and as treatment options improve, patients are less likely to experience adverse reactions.[14,15]
Drug interactions are common because of the idiosyncrasy of these drugs (many are enzyme inductors or inhibitors), and other drugs prescribed to patients. Several studies describe clinically significant drug interactions that could cause adverse reactions in 20%–30% of patients. We do know that protease inhibitors and non-nucleoside reverse transcriptase inhibitors are the antiretroviral subgroups with the most potential interactions.[1,9]
Cuba’s antiretroviral pharmacovigilance program offers a model of active drug surveillance. Latin America and Caribbean nations are making efforts to document these adverse reactions. In Mexico, this documentation is relatively new, with most issues reported reflecting notification quality and patients abandoning treatment due to adverse reactions, leading to an assumption of under-reporting. Although Cuba has an established, globally recognized pharmacovigilance program, under-reporting still exists because of various factors influencing notifications and their quality.[16] According to annual pharmacovigilance reports in Cuba, adverse drug reactions (ADRs) to antiretrovirals represent 0.09% of total notifications about antimicrobials.[17] Among all ADR reports, antimicrobials as a drug group are responsible for the most reports.[18]
Cuban studies on this topic have been limited to one institution or region of the country, and have not considered whether the ADRs were preventable.[19–21] One previous study examined this problem, but included all groups of antivirals, not specifically antiretrovirals.[17] The goal of this study is to describe adverse reactions to antiretrovirals reported in Cuba from 2003 to 2017.
METHODS
Study type A pharmacovigilance study was performed using a case series design. The sample consisted of 352 antiretroviral-drug ADR notifications entered into the National Pharmacovigilance Database (FarmaVigiC) of the National Pharmacovigilance Coordinating Unit (UCNFv)[22] from January 2003 to December 2017.
Study variables The variables were:
- Adverse reaction identified as the main adverse effect, specifying the condition of the patient. We used Uppsala Monitoring Centre adverse reaction terminology.[23]
- Notification year
- Age of children (0–17 years), adults (18–59 years) and persons aged ≥60 years.[22]
- Sex
- Antiretroviral drug
- Frequency: common, uncommon, rare, undescribed. Classified according to the criteria of the Cuban Pharmacovigilance System,[22] which uses the categories and events within each category established for each drug in the National Drug Formulary. The total number of occasional, rare and undescribed adverse reactions were coded together as low-frequency adverse reactions.
- Severity: mild, moderate, severe and fatal. Minor reactions that did not require an antidote, treatment or hospitalization, with easily tolerated symptoms or signs, were classified as mild. Reactions were considered moderate if they required a change in drug treatment or increased observation and caused malaise that interfered with regular activities. They were considered severe when they were life-threatening or caused disability. They were considered fatal when they contributed directly or indirectly to the cause of death.
- Preventable adverse reaction: yes or no, depending on the answer from the Schumock and Thornton preventability algorithm, modified by Otero.[24,25]
- Preventable ADR causes: A cause was considered preventable when there were improper drug instructions, dose, route of administration, intervals of administration or treatment length, a history of adverse effects or allergic reactions to the drug, a drug interaction, or self-medication.
Causality was not among the variables assessed. It was used only as an inclusion criterion for the filters to select the study sample and establish whether ADRs were preventable.
Data collection The information was obtained from FarmaVigiC, which receives spontaneous ADR reports. The database stores information contained in the official template from suspected adverse reaction reports electronically submitted to UCNFv from different levels of the national health system. Reports are made by physicians, nurses and pharmacists caring for patients who take these medications and experience ADRs.
The database was filtered for the field “drug class,” and the term “antiretroviral” and the “suspected drug” was used as the inclusion criterion, which included all drugs classified as antiretrovirals in the Cuban National Drug Formulary and international literature. The database was also filtered for “causality,” where the categories “definite,” “possible” or “probable” were used because they indicate a positive cause-effect relationship between the drug and the ADR. This was done to determine if the ADR was preventable, and was used only as a filter to define the sample. An Excel database was created with the results and named “primary database.” This database stored the remaining fields in FarmaVigiC. The study universe comprised adverse reactions contained in the primary database.
After selecting the ADRs contained in the primary database, the Schumock and Thornton algorithm,[24] modified by Otero,[25] was used to identify preventable ADRs. A “yes” answer to the preventability question indicated that the ADR could have been prevented. Using these databases, a new database was created (“secondary database”), which stored all FarmaVigiC fields. An expert from the UCNFv created both databases without changing variable classifications or assessments.
Potentially preventable reactions were determined based on the information contained in FarmaVigiC, which is validated and verified by trained pharmacovigilance experts. Quality of FarmaVigiC notifications is ensured by regular data review and standardization using different levels of filters according to the quality standards of the Uppsala Monitoring Centre (UMC), the governing body of the WHO Programme for International Drug Monitoring.[22,23] Professionals in Cuban municipalities and provinces receive annual pharmacovigilance training and are supervised by UCNFv specialists.[22]
Data analysis Absolute and relative frequencies and reporting rates of ADRs for antiretrovirals were estimated per year and per million population, by sex and using the population data reported by the National Health Statistics Yearbook.[26] Proportions of patients treated with each antiretroviral were not calculated because the data were not available. The frequency distribution for each drug was estimated based on the total number of notifications.
Ethics The Cuban Ministry of Public Health’s National Drug Division authorized the use of information in FarmaVigiC. Privacy was respected, and no patients who reported antiretroviral ADRs were identified.
RESULTS
During 2003–2017, the new, primary database (filtered FarmaVigiC data) included 352 antiretroviral ADRs. This figure is equivalent to an average reporting rate of 2.09 per million population annually, representing 24.2% of all adverse reactions produced by the antiviral drug class during this period.
Most ADRs were reported from 2009 to 2016, with the greatest share in the final year (85/352; 24.1%). From 2003 to 2005 and in 2007, no antiretroviral ADRs were reported.
Male patients accounted for 77.3% (272/352) and female patients for 22.7% (80/352) of total ADRs notified in the study period. ADR notifications were more common in adults, with higher proportions in men (272/352; 77.3%) than in women (80/352; 22.7%). A single report in children was made, and there were no reports in persons aged >60 years.
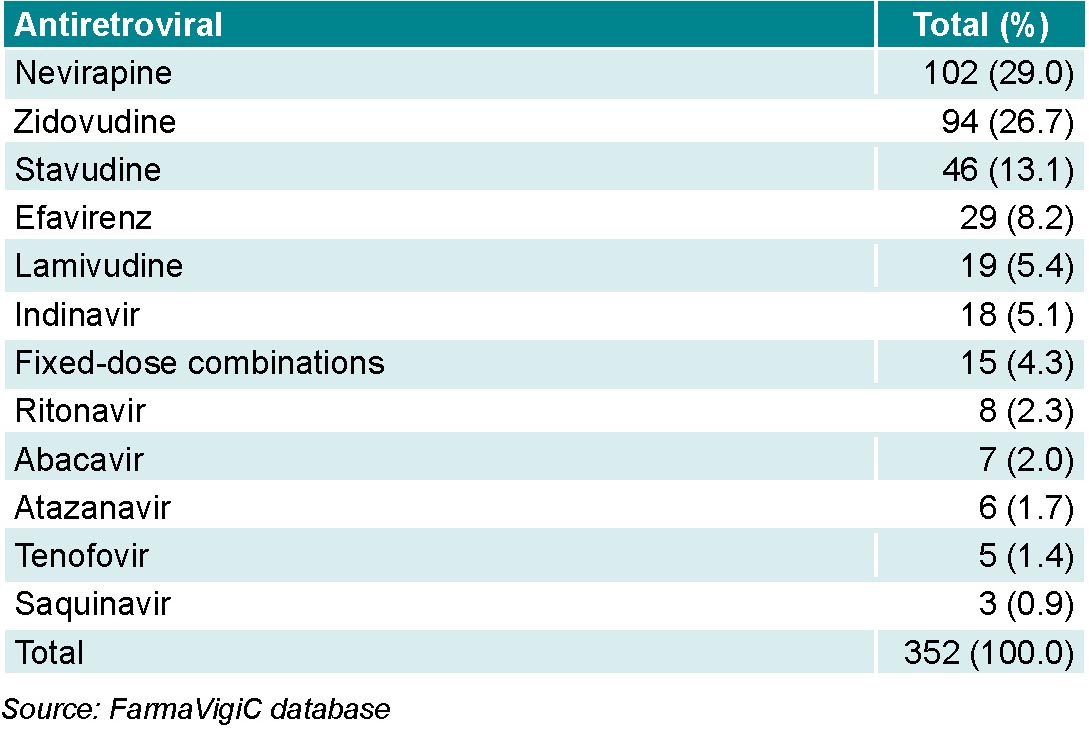
Of the antiretrovirals, nevirapine had the highest number of reports, followed by zidovudine (Table 1).
Formulations containing combinations of antiretrovirals from different groups at fixed doses (Kaletra, Atripla, Truvada, etc.) were responsible for 4.3% (15/352) of reports.
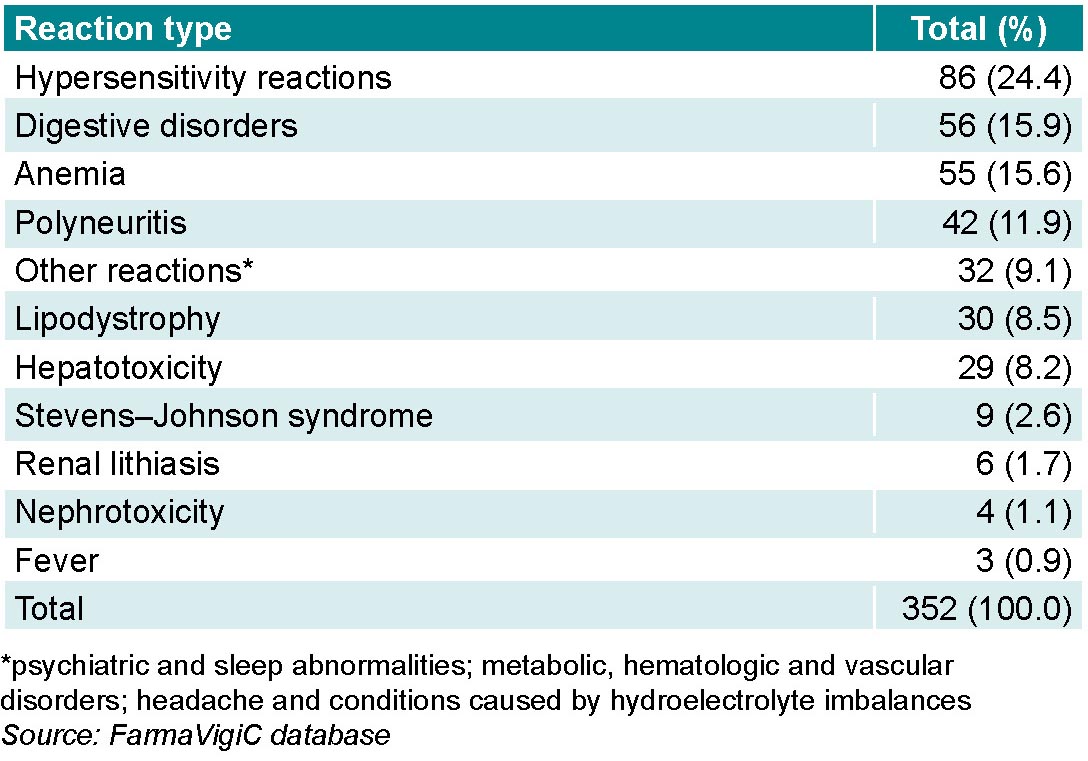
Hypersensitivity reactions accounted for the greatest number of ADRs, followed by digestive disorders and anemia, in which the number of reports differed by a single report. Although hepatotoxicity is not one of the most common ADRs (8.2%; 29/352),it needs to be considered because of its severity (Table 2).
There were 9.1% (32/352) of ADR notifications grouped as “other reactions” because of their infrequent occurrence. Among these were psychiatric and sleep abnormalities and metabolic, hematologic and vascular disorders, as well as headache and conditions caused by hydroelectrolyte imbalances.
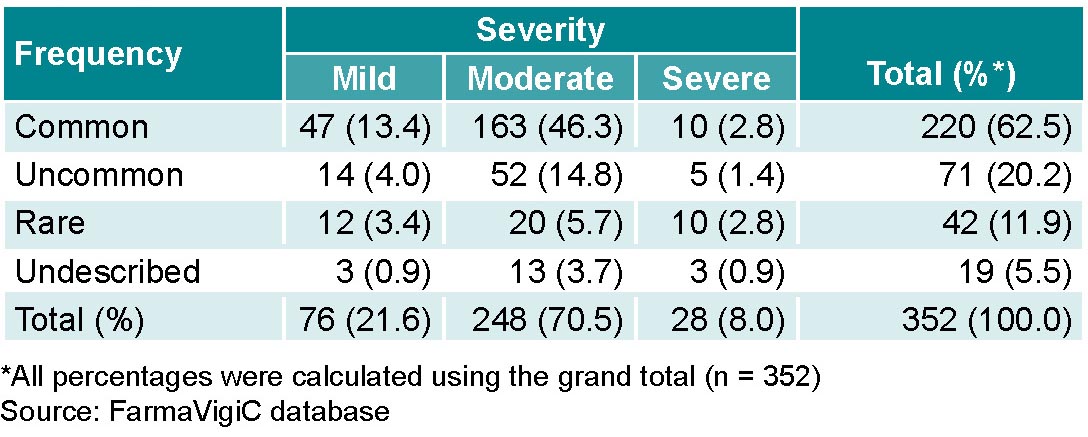
Antiretroviral ADRs were common (62.5%; 220/352). The most common were those of moderate severity (Table 3). Some ADRs, 5.4% (19/352), are not described in the Cuban National Drug Formulary. Any ADRs recorded in the system that do not appear in the National Formulary are reported to the pharmaceutical industry to be evaluated for inclusion in the formulary, depending on their frequency and importance.
No fatal ADRs were reported.
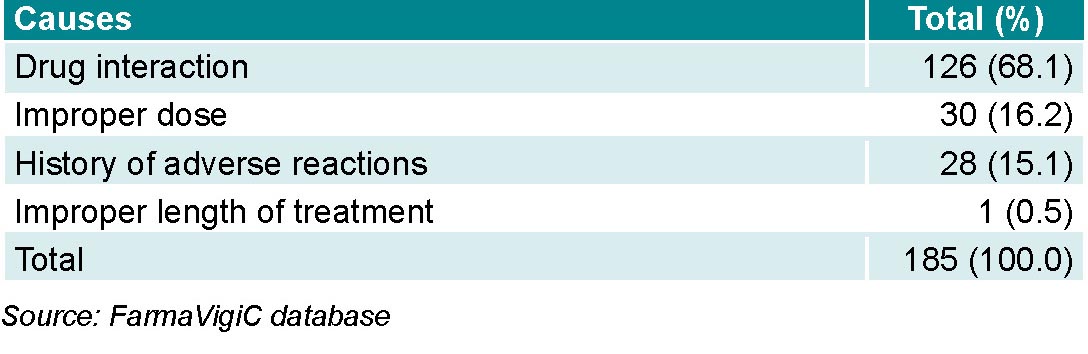
Preventable ADRs made up 52.5% (185/352) of all notifications. Main causes were drug interactions (with drugs in the same drug class or any other that the patient was taking), inappropriate dosage, and a history of ADRs to the suspected drug. Drug interactions alone made up nearly 70% of preventable reactions (Table 4).
DISCUSSION
In Cuba, HIV infection is more common in men than women, in a ratio of 4:1. Men are therefore the largest group of antiretroviral consumers in the country, which would explain the higher percentage of ADRs in males.[9] These results were similar to those obtained in Paraguay, where 62.2% of antiretroviral ADRs were reported in men.[27] A study conducted in Cuba showed that ADRs were more common in adult males.[28]